Evaluation of a novel direct RT-LAMP assay for the detection of SARS-CoV-2 from saliva samples in asymptomatic individuals
- PMID: 35345440
- PMCID: PMC8942569
- DOI: 10.1016/j.jcvp.2022.100074
Evaluation of a novel direct RT-LAMP assay for the detection of SARS-CoV-2 from saliva samples in asymptomatic individuals
Abstract
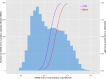
Large scale screening of health care workers and the general population for asymptomatic COVID-19 infection requires modalities that are amenable to testing at scale while retaining acceptable levels of sensitivity and specificity. This study evaluated a novel COVID-19 Direct-RT LAMP assay using saliva samples in asymptomatic individuals by comparison to RT-PCR. Additional studies were performed using VTM collected from routine diagnostic testing. Analytical sensitivity was determined for Direct RT-LAMP assay using the WHO International Standard. Finally, quantified results from RT-PCR testing of 9177 nose and throat swabs obtained from routine diagnostic testing were used to estimate the sensitivity of Direct RT-LAMP using the limit of detection curve obtained from the analytical sensitivity data. Results from saliva testing demonstrated a sensitivity of 40.91% and a specificity of 100% for Direct RT-LAMP. The sensitivity and specificity for nose and throat swabs were 44.85% and 100% respectively. The 95% limit of detection (LOD) for Direct RT-LAMP was log 7.13 IU/ml (95% 6.9-7.5). The estimated sensitivity for Direct-RT LAMP based on the results of 9117 nose and throat swabs was 34% and 45% for saliva and VTM respectively. The overall diagnostic sensitivity of Direct RT-LAMP was low compared to RT-PCR. Testing of nose and throat swabs and estimating the sensitivity based on a large cohort of clinical samples demonstrated similar results. This study highlights the importance of utilising the prospective collection of samples from the intended target population in the assessment of diagnostic sensitivity.
Keywords: Asymptomaitc; COVID-19; Direct RT-LAMP; Real-time PCR; SARS-CoV-2; Saliva; Screening.
Crown Copyright © 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection.mSphere. 2020 Aug 26;5(4):e00808-20. doi: 10.1128/mSphere.00808-20. mSphere. 2020. PMID: 32848011 Free PMC article.
-
RT-LAMP Multicenter Study for SARS-CoV-2 Genome Molecular Detection in Brazilian Swab and Saliva Samples.Diagnostics (Basel). 2023 Jan 6;13(2):210. doi: 10.3390/diagnostics13020210. Diagnostics (Basel). 2023. PMID: 36673025 Free PMC article.
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Detection of SARS-CoV-2 and the L452R spike mutation using reverse transcription loop-mediated isothermal amplification plus bioluminescent assay in real-time (RT-LAMP-BART).PLoS One. 2022 Mar 21;17(3):e0265748. doi: 10.1371/journal.pone.0265748. eCollection 2022. PLoS One. 2022. PMID: 35312732 Free PMC article.
-
Diagnosis of SARS-Cov-2 Infection by RT-PCR Using Specimens Other Than Naso- and Oropharyngeal Swabs: A Systematic Review and Meta-Analysis.Diagnostics (Basel). 2021 Feb 21;11(2):363. doi: 10.3390/diagnostics11020363. Diagnostics (Basel). 2021. PMID: 33670020 Free PMC article. Review.
Cited by
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Evaluation of the artus® Prep&Amp UM RT-PCR for detection of SARS-CoV-2 from nasopharyngeal swabs without prior nucleic acid eluate extraction.J Clin Virol Plus. 2022 Aug;2(3):100098. doi: 10.1016/j.jcvp.2022.100098. Epub 2022 Jul 16. J Clin Virol Plus. 2022. PMID: 35874465 Free PMC article.
-
Reverse transcription loop‑mediated isothermal amplification has a high performance in the detection of SARS‑CoV‑2 in saliva samples and nasal swabs from asymptomatic and symptomatic individuals.Exp Ther Med. 2023 Jul 6;26(2):398. doi: 10.3892/etm.2023.12097. eCollection 2023 Aug. Exp Ther Med. 2023. PMID: 37522063 Free PMC article.
-
Cross comparison of alternative diagnostic protocols including substitution to the clinical sample, RNA extraction method and nucleic acid amplification technology for COVID-19 diagnosis.Front Mol Biosci. 2024 Aug 23;11:1445142. doi: 10.3389/fmolb.2024.1445142. eCollection 2024. Front Mol Biosci. 2024. PMID: 39247206 Free PMC article.
References
-
- NICE . COVID-19 Rapid guideline: Haematopoietic Stem Cell Transplantation. 2020. Overview | COVID-19 rapid guideline: haematopoietic stem cell transplantation | Guidance | NICE.
LinkOut - more resources
Full Text Sources
Miscellaneous
