Electrochemical CO2 reduction toward multicarbon alcohols - The microscopic world of catalysts & process conditions
- PMID: 35345454
- PMCID: PMC8956800
- DOI: 10.1016/j.isci.2022.104010
Electrochemical CO2 reduction toward multicarbon alcohols - The microscopic world of catalysts & process conditions
Abstract
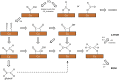
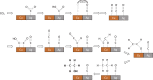
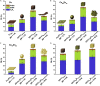
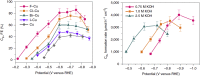
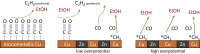
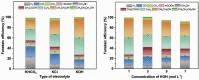
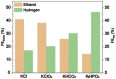
Tackling climate change is one of the undoubtedly most important challenges at the present time. This review deals mainly with the chemical aspects of the current status for converting the greenhouse gas CO2 via electrochemical CO2 reduction reaction (CO2RR) to multicarbon alcohols as valuable products. Feasible reaction routes are presented, as well as catalyst synthesis methods such as electrodeposition, precipitation, or sputtering. In addition, a comprehensive overview of the currently achievable selectivities for multicarbon alcohols in CO2RR is given. It is also outlined to what extent, for example, modifications of the catalyst surfaces or the use of bifunctional compounds the product distribution is shifted. In addition, the influence of varying electrolyte, temperature, and pressure is described and discussed.
Keywords: Catalysis; Electrochemistry.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Abbott A.P., Eardley C.A. Electrochemical reduction of CO2 in a mixed supercritical fluid. J. Phys. Chem. B. 2000;104:775–779.
-
- Abram N., Gattuso J.-P., Prakash A., Cheng L., Chidichimo M.P., Crate S., Enomoto H., Garschagen M., Gruber N., Harper S., et al. Special Report on the Ocean and Cryosphere in a Changing Climate. IPCC; 2019. Framing and context of the report.
-
- Aeshala L.M., Rahman S.U., Verma A. Effect of solid polymer electrolyte on electrochemical reduction of CO2. Sep. Purif. Technol. 2012;94:131–137.
-
- Ahn S.T., Abu-Baker I., Palmore G.R. Electroreduction of CO2 on polycrystalline copper: effect of temperature on product selectivity. Catal. Today. 2017;288:24–29.
-
- Albo J., Perfecto-Irigaray M., Beobide G., Irabien A. Cu/Bi metal-organic framework-based systems for an enhanced electrochemical transformation of CO2 to alcohols. J. CO2 Util. 2019;33:157–165.
Publication types
LinkOut - more resources
Full Text Sources

