Human Amnion-Derived MSCs Alleviate Acute Lung Injury and Hinder Pulmonary Fibrosis Caused by Paraquat in Rats
- PMID: 35345827
- PMCID: PMC8957415
- DOI: 10.1155/2022/3932070
Human Amnion-Derived MSCs Alleviate Acute Lung Injury and Hinder Pulmonary Fibrosis Caused by Paraquat in Rats
Abstract
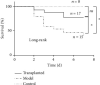
Methods: First, the purity of hAD-MSCs was determined by morphological observation and FCM, and the effects on the survival of paraquat-poisoned Sprague-Dawley rats were observed. All rats were randomly divided into three groups, defined as the sham control group (n = 8), model group (n = 15), and hAD-MSC-transplanted group (n = 17). Pneumonocyte damage and inflammatory cell infiltration were investigated in the three groups of rats, untreated control, paraquat only, and paraquat+hAD-MSC transplanted, using H&E staining. Fibrosis was investigated in three groups of rats using Masson's trichrome staining and Sirius red staining. The profibrotic factor TGF-β1, the composition of fibrotic collagen HYP, and the hAD-MSC-secreted immunosuppressive factor HLA-G5 in serum were investigated in the three groups of rats using ELISA. Furthermore, the distribution of hAD-MSCs was investigated in the three groups of rats using immunohistochemistry and hematoxylin staining.
Results: The hAD-MSCs exhibited typical hallmarks of MSCs, improved the state of being and survival of paraquat-poisoned rats, reduced both lung injury and inflammation, and inhibited the progression of pulmonary fibrosis by decreasing the deposition of collagen and the secretion of both TGF-β1 and HYP. The hAD-MSCs could survive in damaged lungs and secreted appropriate amounts of HLA-G5 into the serum.
Conclusion: The obtained results indicate that hAD-MSCs used to treat paraquat-induced lung injury may work through anti-inflammatory and immunosuppressive pathways and the downregulation of profibrotic elements. This study suggests that the transplantation of hAD-MSCs is a promising therapeutic approach for the treatment of paraquat-intoxicated patients.
Copyright © 2022 Liming Gong et al.
Conflict of interest statement
Our discovery has been granted a Chinese invention patent and has no conflict of interest with any other entity or individual.
Figures






Similar articles
-
[Effect of bone marrow mesenchymal stem cells on paraquat-induced pulmonary fibrosis in rats].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020 May 20;38(5):332-338. doi: 10.3760/cma.j.cn121094-20190711-00214. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2020. PMID: 32536068 Chinese.
-
Effects of rapamycin against paraquat-induced pulmonary fibrosis in mice.J Zhejiang Univ Sci B. 2015 Jan;16(1):52-61. doi: 10.1631/jzus.B1400229. J Zhejiang Univ Sci B. 2015. PMID: 25559956 Free PMC article.
-
Losartan attenuates paraquat-induced pulmonary fibrosis in rats.Hum Exp Toxicol. 2015 May;34(5):497-505. doi: 10.1177/0960327114543840. Epub 2014 Sep 17. Hum Exp Toxicol. 2015. PMID: 25233898
-
Protective effect of ambroxol against paraquat-induced pulmonary fibrosis in rats.Intern Med. 2011;50(18):1879-87. doi: 10.2169/internalmedicine.50.5407. Epub 2011 Sep 15. Intern Med. 2011. PMID: 21921364
-
Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response.Life Sci. 2020 Feb 15;243:117290. doi: 10.1016/j.lfs.2020.117290. Epub 2020 Jan 8. Life Sci. 2020. PMID: 31923420
Cited by
-
Human amnion-derived mesenchymal stem cells attenuate acute lung injury in two different acute lung injury mice models.Front Pharmacol. 2023 Jun 14;14:1149659. doi: 10.3389/fphar.2023.1149659. eCollection 2023. Front Pharmacol. 2023. PMID: 37388446 Free PMC article.
-
The Role of Mesenchymal Stem Cells in Modulating the Breast Cancer Microenvironment.Cell Transplant. 2023 Jan-Dec;32:9636897231220073. doi: 10.1177/09636897231220073. Cell Transplant. 2023. PMID: 38135917 Free PMC article. Review.
References
-
- Afzali S., et al. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Archives of Iranian Medicine . 2008;11(4):387–391. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

