Chicago sky blue 6B inhibits α-synuclein aggregation and propagation
- PMID: 35346306
- PMCID: PMC8962151
- DOI: 10.1186/s13041-022-00913-y
Chicago sky blue 6B inhibits α-synuclein aggregation and propagation
Abstract
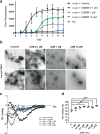
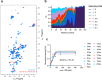
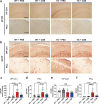
Abnormal deposition of α-synuclein aggregates in Lewy bodies and Lewy neurites is the hallmark lesion in Parkinson's disease (PD). These aggregates, thought to be the culprit of disease pathogenesis, spread throughout the brain as the disease progresses. Agents that inhibit α-synuclein aggregation and/or spread of aggregates would thus be candidate disease-modifying drugs. Here, we found that Chicago sky blue 6B (CSB) may be such a drug, showing that it inhibits α-synuclein aggregation and cell-to-cell propagation in both in vitro and in vivo models of synucleinopathy. CSB inhibited the fibrillation of α-synuclein in a concentration-dependent manner through direct binding to the N-terminus of α-synuclein. Furthermore, both seeded polymerization and cell-to-cell propagation of α-synuclein were inhibited by CSB treatment. Notably, CSB alleviated behavioral deficits and neuropathological features, such as phospho-α-synuclein and astrogliosis, in A53T α-synuclein transgenic mice. These results indicate that CSB directly binds α-synuclein and inhibits its aggregation, thereby blocking α-synuclein cell-to-cell propagation.
Keywords: Aggregate propagation; Chicago sky blue 6B; Parkinson’s disease; Protein aggregation; α-synuclein.
© 2022. The Author(s).
Conflict of interest statement
S.-J.L is a founder and CEO of Neuramedy Co., Ltd. Other authors declare that they have no competing interests.
Figures


Similar articles
-
14-3-3 Proteins Reduce Cell-to-Cell Transfer and Propagation of Pathogenic α-Synuclein.J Neurosci. 2018 Sep 19;38(38):8211-8232. doi: 10.1523/JNEUROSCI.1134-18.2018. Epub 2018 Aug 9. J Neurosci. 2018. PMID: 30093536 Free PMC article.
-
Naturally occurring antibodies isolated from PD patients inhibit synuclein seeding in vitro and recognize Lewy pathology.Acta Neuropathol. 2019 May;137(5):825-836. doi: 10.1007/s00401-019-01974-5. Epub 2019 Feb 25. Acta Neuropathol. 2019. PMID: 30805666 Free PMC article.
-
Pathological biochemistry of alpha-synucleinopathy.Neuropathology. 2007 Oct;27(5):474-8. doi: 10.1111/j.1440-1789.2007.00785.x. Neuropathology. 2007. Retraction in: Neuropathology. 2012 Jun;32(3):318. doi: 10.1111/j.1440-1789.2012.01313.x. PMID: 18018483 Retracted.
-
Clearance Pathways for α-Synuclein in Parkinson's Disease.J Neurochem. 2025 Jun;169(6):e70124. doi: 10.1111/jnc.70124. J Neurochem. 2025. PMID: 40509661 Free PMC article. Review.
-
Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson's Disease?Biomolecules. 2015 Jul 16;5(3):1467-79. doi: 10.3390/biom5031467. Biomolecules. 2015. PMID: 26193328 Free PMC article. Review.
Cited by
-
TNF-α disrupts the malate-aspartate shuttle, driving metabolic rewiring in iPSC-derived enteric neural lineages from Parkinson's Disease patients.bioRxiv [Preprint]. 2025 Mar 26:2025.03.25.644826. doi: 10.1101/2025.03.25.644826. bioRxiv. 2025. PMID: 40196623 Free PMC article. Preprint.
-
Select azo compounds post-translationally modulate HTRA1 abundance and activity potentially through interactions at the trimer interface.bioRxiv [Preprint]. 2025 May 16:2025.05.13.651909. doi: 10.1101/2025.05.13.651909. bioRxiv. 2025. Update in: ACS Chem Biol. 2025 Aug 15;20(8):1849-1862. doi: 10.1021/acschembio.4c00818. PMID: 40463243 Free PMC article. Updated. Preprint.
-
Benzbromarone interferes with the interaction between Hsp90 and Aha1 by interacting with both of them.Commun Biol. 2025 May 16;8(1):761. doi: 10.1038/s42003-025-08189-3. Commun Biol. 2025. PMID: 40379881 Free PMC article.
-
A Biomimetic Multiparametric Assay to Characterise Anti-Amyloid Drugs.Int J Mol Sci. 2023 Nov 30;24(23):16982. doi: 10.3390/ijms242316982. Int J Mol Sci. 2023. PMID: 38069305 Free PMC article.
References
-
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/WNL.47.5.1113. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

