Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial
- PMID: 35346319
- PMCID: PMC8958810
- DOI: 10.1186/s13054-022-03950-0
Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: analysis of a randomized controlled trial
Abstract
Background: Awake prone positioning (APP) improves oxygenation in coronavirus disease (COVID-19) patients and, when successful, may decrease the risk of intubation. However, factors associated with APP success remain unknown. In this secondary analysis, we aimed to assess whether APP can reduce intubation rate in patients with COVID-19 and to focus on the factors associated with success.
Methods: In this multicenter randomized controlled trial, conducted in three high-acuity units, we randomly assigned patients with COVID-19-induced acute hypoxemic respiratory failure (AHRF) requiring high-flow nasal cannula (HFNC) oxygen to APP or standard care. Primary outcome was intubation rate at 28 days. Multivariate analyses were performed to identify the predictors associated to treatment success (survival without intubation).
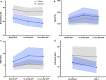
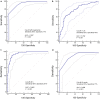
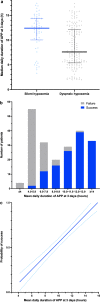
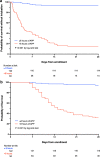
Results: Among 430 patients randomized, 216 were assigned to APP and 214 to standard care. The APP group had a lower intubation rate (30% vs 43%, relative risk [RR] 0.70; CI95 0.54-0.90, P = 0.006) and shorter hospital length of stay (11 interquartile range [IQR, 9-14] vs 13 [IQR, 10-17] days, P = 0.001). A respiratory rate ≤ 25 bpm at enrollment, an increase in ROX index > 1.25 after first APP session, APP duration > 8 h/day, and a decrease in lung ultrasound score ≥ 2 within the first 3 days were significantly associated with treatment success for APP.
Conclusion: In patients with COVID-19-induced AHRF treated by HFNC, APP reduced intubation rate and improved treatment success. A longer APP duration is associated with APP success, while the increase in ROX index and decrease in lung ultrasound score after APP can also help identify patients most likely to benefit.
Trial registration: This study was retrospectively registered in ClinicalTrials.gov at July 20, 2021. Identification number NCT04477655. https://clinicaltrials.gov/ct2/show/NCT04477655?term=PRO-CARF&draw=2&rank=1.
Keywords: Acute hypoxemic respiratory failure; Awake prone positioning; COVID-19; Intubation.
© 2022. The Author(s).
Conflict of interest statement
JL discloses research funding from Fisher & Paykel Healthcare Ltd, Aerogen Ltd, and Rice Foundation, and speaker fees from American Association for Respiratory Care, Aerogen Ltd, Heyer Ltd, and Fisher & Paykel Healthcare Ltd. IP discloses a research grant and speaker fees from Fisher & Paykel Healthcare Ltd. YP discloses research support from Fisher & Paykel Healthcare Ltd. OR discloses a research grant from Hamilton Medical and speaker fees from Hamilton Medical, Ambu and Aerogen Ltd, and non-financial research support from Timpel and Masimo Corporation. His institution received fees for consultancy from Hamilton Medical. DV discloses research funding from Teleflex Medical, Inc. and Rice Foundation, and speaker fees from Theravance Biopharma. SE discloses consultancies from Aerogen Ltd, research support from Aerogen Ltd, Fisher & Paykel Healthcare Ltd, Hamilton medical, travel reimbursements from Aerogen Ltd and Fisher & Paykel Healthcare Ltd. JGL discloses consulting fees from Baxter Healthcare and Glaxosmithkline. All other authors have no conflict of interest to disclose.
Figures
References
-
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, the Northwell COVID-19 Research Consortium. Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. - DOI - PMC - PubMed
-
- Cueto-Manzano AM, Espinel-Bermúdez MC, Hernández-González SO, Rojas-Campos E, Nava-Zavala AH, Fuentes-Orozco C, Balderas-Peña LMA, González-Ojeda A, Cortes-Sanabria L, Mireles-Ramírez MA, et al. Risk factors for mortality of adult patients with COVID-19 hospitalised in an emerging country: a cohort study. BMJ Open. 2021;11(7):e050321. doi: 10.1136/bmjopen-2021-050321. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

