Cilia-derived vesicles: An ancient route for intercellular communication
- PMID: 35346578
- PMCID: PMC9378432
- DOI: 10.1016/j.semcdb.2022.03.014
Cilia-derived vesicles: An ancient route for intercellular communication
Abstract
Extracellular vesicles (EVs) provide a mechanism for intercellular communication that transports complex signals in membrane delimited structures between cells, tissues and organisms. Cells secrete EVs of various subtypes defined by the pathway leading to release and by the pathological condition of the cell. Cilia are evolutionarily conserved organelles that can act as sensory structures surveilling the extracellular environment. Here we discuss the secretory functions of cilia and their biological implications. Studies in multiple species - from the nematode Caenorhabditis elegans and the chlorophyte alga Chlamydomonas reinhardtii to mammals - have revealed that cilia shed bioactive EVs (ciliary EVs or ectosomes) by outward budding of the ciliary membrane. The content of ciliary EVs is distinct from that of other vesicles released by cells. Peptides regulate numerous aspects of metazoan physiology and development through evolutionarily conserved mechanisms. Intriguingly, cilia-derived vesicles have recently been found to mediate peptidergic signaling. C. reinhardtii releases the peptide α-amidating enzyme (PAM), bioactive amidated products and components of the peptidergic signaling machinery in ciliary EVs in a developmentally regulated manner. Considering the origin of cilia in early eukaryotes, it is likely that release of peptidergic signals in ciliary EVs represents an alternative and ancient mode of regulated secretion that cells can utilize in the absence of dedicated secretory granules.
Keywords: Amidation; Chlamydomonas; Cilia; Ectosome; Peptidergic Signaling.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no competing interests.
Figures


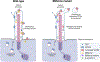
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

