Understanding the physical basis of memory: Molecular mechanisms of the engram
- PMID: 35346687
- PMCID: PMC9065729
- DOI: 10.1016/j.jbc.2022.101866
Understanding the physical basis of memory: Molecular mechanisms of the engram
Erratum in
-
Correction: Understanding the physical basis of memory: Molecular mechanisms of the engram.J Biol Chem. 2023 Aug;299(8):105070. doi: 10.1016/j.jbc.2023.105070. Epub 2023 Jul 25. J Biol Chem. 2023. PMID: 37499591 Free PMC article. No abstract available.
Abstract
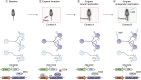
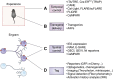
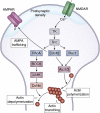
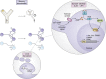
Memory, defined as the storage and use of learned information in the brain, is necessary to modulate behavior and critical for animals to adapt to their environments and survive. Despite being a cornerstone of brain function, questions surrounding the molecular and cellular mechanisms of how information is encoded, stored, and recalled remain largely unanswered. One widely held theory is that an engram is formed by a group of neurons that are active during learning, which undergoes biochemical and physical changes to store information in a stable state, and that are later reactivated during recall of the memory. In the past decade, the development of engram labeling methodologies has proven useful to investigate the biology of memory at the molecular and cellular levels. Engram technology allows the study of individual memories associated with particular experiences and their evolution over time, with enough experimental resolution to discriminate between different memory processes: learning (encoding), consolidation (the passage from short-term to long-term memories), and storage (the maintenance of memory in the brain). Here, we review the current understanding of memory formation at a molecular and cellular level by focusing on insights provided using engram technology.
Keywords: code; engram; memory; molecular mechanism; plasticity; synapse.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Kandel E.R., Mack S., Jessell T.M., Schwartz J.H., Siegelbaum S.A., Hudspeth A.J. McGraw-Hill Education; New York, NY: 2013. Principles of Neural Science, Fifth Edition.
-
- Tulving E. Episodic memory: From mind to brain. Annu. Rev. Psychol. 2002;53:1–25. - PubMed
-
- Dudai Y., Roediger H.L., Tulving E. In: Science of Memory: Concepts. Roedinger H.L., Dudai Y., Fitzpatrick S.M., editors. Oxford University Press; New York, NY: 2007. Memory concepts; pp. 1–9.
-
- McGaugh J.L. Memory - a century of consolidation. Science. 2000;287:248–251. - PubMed
-
- Schacter D.L., Tulving E. MIT Press; Cambridge, MA: 2020. Memory Systems 1994.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

