A genetically targeted sensor reveals spatial and temporal dynamics of acrosomal calcium and sperm acrosome exocytosis
- PMID: 35346690
- PMCID: PMC9046242
- DOI: 10.1016/j.jbc.2022.101868
A genetically targeted sensor reveals spatial and temporal dynamics of acrosomal calcium and sperm acrosome exocytosis
Abstract
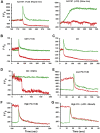
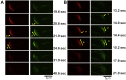
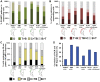
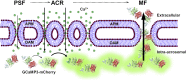
Secretion of the acrosome, a single vesicle located rostrally in the head of a mammalian sperm, through a process known as "acrosome exocytosis" (AE), is essential for fertilization. However, the mechanisms leading to and regulating this complex process are controversial. In particular, poor understanding of Ca2+ dynamics between sperm subcellular compartments and regulation of membrane fusion mechanisms have led to competing models of AE. Here, we developed a transgenic mouse expressing an Acrosome-targeted Sensor for Exocytosis (AcroSensE) to investigate the spatial and temporal Ca2+ dynamics in AE in live sperm. AcroSensE combines a genetically encoded Ca2+ indicator (GCaMP) fused with an mCherry indicator to spatiotemporally resolve acrosomal Ca2+ rise (ACR) and membrane fusion events, enabling real-time study of AE. We found that ACR is dependent on extracellular Ca2+ and that ACR precedes AE. In addition, we show that there are intermediate steps in ACR and that AE correlates better with the ACR rate rather than absolute Ca2+ amount. Finally, we demonstrate that ACR and membrane fusion progression kinetics and spatial patterns differ with different stimuli and that sites of initiation of ACR and sites of membrane fusion do not always correspond. These findings support a model involving functionally redundant pathways that enable a highly regulated, multistep AE in heterogeneous sperm populations, unlike the previously proposed "acrosome reaction" model.
Keywords: acrosome exocytosis; fertilization; microscopy; mouse; sperm.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest A. J. T. discloses that he is a founder and an officer of Androvia LifeSciences, LLC, a biotechnology company investigating solutions for male infertility, in which he holds a minor equity stake. There are no conflicts with the current work. All other coauthors declare that they have no conflicts of interest with the contents of this article.
Figures





References
-
- Kim K.S., Cha M.C., Gerton G.L. Mouse sperm protein sp56 is a component of the acrosomal matrix. Biol. Reprod. 2001;64:36–43. - PubMed
-
- Kim K.S., Gerton G.L. Differential release of soluble and matrix components: Evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev. Biol. 2003;264:141–152. - PubMed
-
- Baibakov B., Gauthier L., Talbot P., Rankin T.L., Dean J. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development. 2007;134:933–943. - PubMed
-
- Hino T., Muro Y., Tamura-Nakano M., Okabe M., Tateno H., Yanagimachi R. The behavior and acrosomal status of mouse spermatozoa in vitro, and within the oviduct during fertilization after natural mating. Biol. Reprod. 2016;95:50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

