The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs
- PMID: 35346768
- PMCID: PMC9447088
- DOI: 10.1016/j.jconrel.2022.03.046
The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs
Abstract
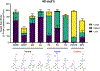
The broad clinical application of mRNA therapeutics has been hampered by a lack of delivery vehicles that induce protein expression in extrahepatic organs and tissues. Recently, it was shown that mRNA delivery to the spleen or lungs is possible upon the addition of a charged lipid to a standard four-component lipid nanoparticle formulation. This approach, while effective, further complicates an already complex drug formulation and has the potential to slow regulatory approval and adversely impact manufacturing processes. We were thus motivated to maintain a four-component nanoparticle system while achieving shifts in tropism. To that end, we replaced the standard helper lipid in lipidoid nanoparticles, DOPE, with one of eight alternatives. These lipids included the neutral lipids, DOPC, sphingomyelin, and ceramide; the anionic lipids, phosphatidylserine (PS), phosphatidylglycerol, and phosphatidic acid; and the cationic lipids, DOTAP and ethyl phosphatidylcholine. While neutral helper lipids maintained protein expression in the liver, anionic and cationic lipids shifted protein expression to the spleen and lungs, respectively. For example, replacing DOPE with DOTAP increased positive LNP surface charge at pH 7 by 5-fold and altered the ratio of liver to lung protein expression from 36:1 to 1:56. Similarly, replacing DOPE with PS reduced positive charge by half and altered the ratio of liver to spleen protein expression from 8:1 to 1:3. Effects were consistent across ionizable lipidoid chemistries. Regarding mechanism, nanoparticles formulated with neutral and anionic helper lipids best transfected epithelial and immune cells, respectively. Further, the lung-tropic effect of DOTAP was linked to reduced immune cell infiltration of the lungs compared to neutral or anionic lipids. Together, these data show that intravenous non-hepatocellular mRNA delivery is readily achievable while maintaining a four-component formulation with modified helper lipid chemistry.
Keywords: Charge; Extrahepatic; Helper lipids; Lipid nanoparticles; Targeted delivery; mRNA delivery.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



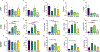

Similar articles
-
Branched-Tail Lipid Nanoparticles for Intravenous mRNA Delivery to Lung Immune, Endothelial, and Alveolar Cells in Mice.Adv Healthc Mater. 2024 Sep;13(22):e2400225. doi: 10.1002/adhm.202400225. Epub 2024 Jul 2. Adv Healthc Mater. 2024. PMID: 38888972
-
Bile acid-containing lipid nanoparticles enhance extrahepatic mRNA delivery.Theranostics. 2024 Jan 1;14(1):1-16. doi: 10.7150/thno.89913. eCollection 2024. Theranostics. 2024. PMID: 38164140 Free PMC article.
-
Helper lipid structure influences protein adsorption and delivery of lipid nanoparticles to spleen and liver.Biomater Sci. 2021 Feb 21;9(4):1449-1463. doi: 10.1039/d0bm01609h. Epub 2021 Jan 6. Biomater Sci. 2021. PMID: 33404020 Free PMC article.
-
The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery.Adv Drug Deliv Rev. 2016 Apr 1;99(Pt A):129-137. doi: 10.1016/j.addr.2016.01.022. Epub 2016 Feb 18. Adv Drug Deliv Rev. 2016. PMID: 26900977 Review.
-
Chemistry of Lipid Nanoparticles for RNA Delivery.Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review.
Cited by
-
Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism.Nat Commun. 2023 Nov 1;14(1):6983. doi: 10.1038/s41467-023-42672-x. Nat Commun. 2023. PMID: 37914693 Free PMC article.
-
Transition Temperature-Guided Design of Lipid Nanoparticles for Effective mRNA Delivery.ACS Appl Mater Interfaces. 2025 May 14;17(19):28012-28024. doi: 10.1021/acsami.5c06464. Epub 2025 May 5. ACS Appl Mater Interfaces. 2025. PMID: 40325908 Free PMC article.
-
Current research trends of nanomedicines.Acta Pharm Sin B. 2023 Nov;13(11):4391-4416. doi: 10.1016/j.apsb.2023.05.018. Epub 2023 May 20. Acta Pharm Sin B. 2023. PMID: 37969727 Free PMC article. Review.
-
Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity.Vaccines (Basel). 2024 Feb 12;12(2):186. doi: 10.3390/vaccines12020186. Vaccines (Basel). 2024. PMID: 38400169 Free PMC article. Review.
-
Development of Lipid Nanoparticle Formulation for the Repeated Administration of mRNA Therapeutics.Biomater Res. 2024 May 22;28:0017. doi: 10.34133/bmr.0017. eCollection 2024. Biomater Res. 2024. PMID: 38779139 Free PMC article.
References
-
- Basha G, Novobrantseva T, Rosin N, Tam Y, Hafez I, Wong M, Sugo T, Ruda V, Qin J, Klebanov B, Ciufolini M, Akinc A, Tam Y, Hope M, Cullis P, Influence of Cationic Lipid Composition on Gene Silencing Properties of Lipid Nanoparticle Formulations of siRNA in Antigen-Presenting Cells, Molecular therapy : the journal of the American Society of Gene Therapy, 19 (2011) 2186–2200. - PMC - PubMed
-
- Miller JB, Kos P, Tieu V, Zhou K, Siegwart DJ, Development of Cationic Quaternary Ammonium Sulfonamide Amino Lipids for Nucleic Acid Delivery, ACS Appl Mater Interfaces, 10 (2018) 2302–2311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

