eEF2K promotes PD-L1 stabilization through inactivating GSK3β in melanoma
- PMID: 35347072
- PMCID: PMC8961175
- DOI: 10.1136/jitc-2021-004026
eEF2K promotes PD-L1 stabilization through inactivating GSK3β in melanoma
Abstract
Background: Immune checkpoint blockade (ICB) targeting programmed death ligand-1 (PD-L1)/programmed cell death protein-1 (PD-1) pathway has become an attractive strategy for cancer treatment; however, unsatisfactory efficacy has limited its clinical benefits. Therefore, a more comprehensive understanding of the regulation of PD-L1 expression is essential for developing more effective cancer immunotherapy. Recent studies have revealed the important roles of eukaryotic elongation factor 2 kinase (eEF2K) in promoting epithelial-mesenchymal transition (EMT), angiogenesis, tumor cell migration and invasion; nevertheless, the exact role of eEF2K in the regulation of tumor immune microenvironment (TIME) remains largely unknown.
Methods: In this study, we used a cohort of 38 patients with melanoma who received anti-PD-1 treatment to explore the association between eEF2K expression and immunotherapy efficacy against melanoma. Immunoprecipitation-mass spectrometry analysis and in vitro assays were used to examine the role and molecular mechanism of eEF2K in regulating PD-L1 expression. We also determined the effects of eEF2K on tumor growth and cytotoxicity of CD8+ T cells in TIME in a mouse melanoma model. We further investigated the efficacy of the eEF2K inhibition in combination with anti-PD-1 treatment in vivo.
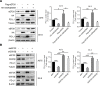
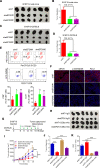
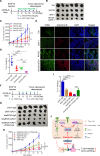
Results: High eEF2K expression is correlated with better therapeutic response and longer survival in patients with melanoma treated with PD-1 monoclonal antibody (mAb). Moreover, eEF2K protein expression is positively correlated with PD-L1 protein expression. Mechanistically, eEF2K directly bound to and inactivated glycogen synthase kinase 3 beta (GSK3β) by phosphorylating it at serine 9 (S9), leading to PD-L1 protein stabilization and upregulation, and subsequently tumor immune evasion. Knockdown of eEF2K decreased PD-L1 expression and enhanced CD8+ T cell activity, thus dramatically attenuating murine B16F10 melanoma growth in vivo. Clinically, p-GSK3β/S9 expression is positively correlated with the expressions of eEF2K and PD-L1, and the response to anti-PD-1 immunotherapy. Furthermore, eEF2K inhibitor, NH125 treatment or eEF2K knockdown enhanced the efficacy of PD-1 mAb therapy in a melanoma mouse model.
Conclusions: Our results suggest that eEF2K may serve as a biomarker for predicting therapeutic response and prognosis in patients receiving anti-PD-1 therapy, reveal a vital role of eEF2K in regulating TIME by controlling PD-L1 expression and provide a potential combination therapeutic strategy of eEF2K inhibition with ICB therapy.
Keywords: biomarkers, tumor; drug therapy, combination; immunotherapy; melanoma; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
