C-reactive protein cut-off for early tocilizumab and dexamethasone prescription in hospitalized patients with COVID-19
- PMID: 35347166
- PMCID: PMC8960074
- DOI: 10.1038/s41598-022-08882-x
C-reactive protein cut-off for early tocilizumab and dexamethasone prescription in hospitalized patients with COVID-19
Abstract
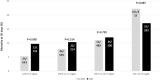
Dexamethasone and tocilizumab have been associated with reduction in mortality, however, the beneficial effect is not for all patients and the impact on viral replication is not well defined. We hypostatized that C-reactive protein (CRP) could help in the identification of patients requiring anti-inflammatory therapy. Patients admitted for > 48 h in our hospital for a confirmed or suspected infection by SARS-CoV-2 from February 2020 to February 2021 were retrospectively evaluated. The primary outcome was mortality at 30 days. Demographics and the most relevant variables related with the outcome were included. CRP was stratified by percentiles. Univariate and multivariate analysis were performed. A total of 3218 patients were included with a median (IQR) age of 66 (74-78) years and 58.9% were males. The rate of intensive care unit admission was 24.4% and the 30-day mortality rate was 11.8%. Within the first 5 days from admission, 1018 (31.7%) patients received dexamethasone and 549 tocilizumab (17.1%). The crude analysis showed a mortality reduction in patients receiving dexamethasone when CRP was > 13.75 mg/dL and > 3.5 mg/dL for those receiving tocilizumab. Multivariate analysis identified the interaction of CRP > 13.75 mg/dL with dexamethasone (OR 0.57; CI 95% 0.37-0.89, P = 0014) and CRP > 3.5 mg/dL with tocilizumab (0.65; CI95%:0.44-0.95, P = 0.029) as independent predictors of mortality. Our results suggest that dexamethasone and tocilizumab are associated with a reduction in mortality when prescribed to patients with a certain inflammatory activity assessed by C-reactive protein.
© 2022. The Author(s).
Conflict of interest statement
CGV has received honoraria for talks on behalf of Gilead Science, MSD, Novartis, Pfizer, Jannsen, and Lilly, as well as a grant from Gilead Science and MSD. PPA has received honoraria for talks on behalf of Gilead Science and MSD. JM has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, and Angellini. AS has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer, Novartis, Gilead, Menarini, and Angellini, as well as grant support from Pfizer and Gilead. MT has received grants from Janssen, Gilead, ViiV and Merck Sharp and Dohme. LM has received honoraria for talks on behalf of Merck Sharp and Dohme, Pfizer and Angellini. PC has received honoraria for talks on behalf of Merck Sharp and Dohme, has participated in Advisory Boards for Gilead and Alexion, and has received grant support from Pfizer and Gilead. Other authors do not declare conflict of interest.
Figures

References
-
- Hopkins, J. COVID-19 dashboard: By the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

