Intra- and interpopulation transposition of mobile genetic elements driven by antibiotic selection
- PMID: 35347261
- PMCID: PMC12520065
- DOI: 10.1038/s41559-022-01705-2
Intra- and interpopulation transposition of mobile genetic elements driven by antibiotic selection
Abstract
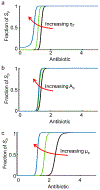
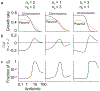
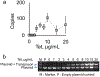
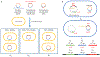
The spread of genes encoding antibiotic resistance is often mediated by horizontal gene transfer (HGT). Many of these genes are associated with transposons, a type of mobile genetic element that can translocate between the chromosome and plasmids. It is widely accepted that the translocation of antibiotic resistance genes onto plasmids potentiates their spread by HGT. However, it is unclear how this process is modulated by environmental factors, especially antibiotic treatment. To address this issue, we asked whether antibiotic exposure would select for the transposition of resistance genes from chromosomes onto plasmids and, if so, whether antibiotic concentration could tune the distribution of resistance genes between chromosomes and plasmids. We addressed these questions by analysing the transposition dynamics of synthetic and natural transposons that encode resistance to different antibiotics. We found that stronger antibiotic selection leads to a higher fraction of cells carrying the resistance on plasmids because the increased copy number of resistance genes on multicopy plasmids leads to higher expression of those genes and thus higher cell survival when facing antibiotic selection. Once they have transposed to plasmids, antibiotic resistance genes are primed for rapid spread by HGT. Our results provide quantitative evidence for a mechanism by which antibiotic selection accelerates the spread of antibiotic resistance in microbial communities.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests statement
None of the authors have competing financial or non-financial interests.
Figures
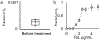

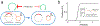





Comment in
-
The journey of bacterial genes.Nat Ecol Evol. 2022 May;6(5):498-499. doi: 10.1038/s41559-022-01713-2. Nat Ecol Evol. 2022. PMID: 35347260 No abstract available.
References
-
- Stokes HW & Gillings MR Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS microbiology reviews 35, 790–819 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

