Fast and selective reduction of nitroarenes under visible light with an earth-abundant plasmonic photocatalyst
- PMID: 35347273
- PMCID: PMC9117130
- DOI: 10.1038/s41565-022-01087-3
Fast and selective reduction of nitroarenes under visible light with an earth-abundant plasmonic photocatalyst
Abstract
Reduction of nitroaromatics to the corresponding amines is a key process in the fine and bulk chemicals industry to produce polymers, pharmaceuticals, agrochemicals and dyes. However, their effective and selective reduction requires high temperatures and pressurized hydrogen and involves noble metal-based catalysts. Here we report on an earth-abundant, plasmonic nano-photocatalyst, with an excellent reaction rate towards the selective hydrogenation of nitroaromatics. With solar light as the only energy input, the chalcopyrite catalyst operates through the combined action of hot holes and photothermal effects. Ultrafast laser transient absorption and light-induced electron paramagnetic resonance spectroscopies have unveiled the energy matching of the hot holes in the valence band of the catalyst with the frontier orbitals of the hydrogen and electron donor, via a transient coordination intermediate. Consequently, the reusable and sustainable copper-iron-sulfide (CuFeS2) catalyst delivers previously unattainable turnover frequencies, even in large-scale reactions, while the cost-normalized production rate stands an order of magnitude above the state of the art.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
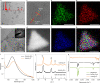
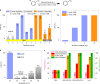
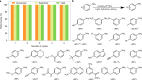

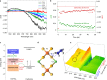
References
-
- Ross, J. R. H. Contemporary Catalysis: Fundamentals and Current Applications (Elsevier, 2019).
-
- Blaser HU, et al. Selective hydrogenation for fine chemicals: recent trends and new developments. Adv. Synth. Catal. 2003;345:103–151. doi: 10.1002/adsc.200390000. - DOI
Grants and funding
- CZ.02.2.69/0.0/0.0/20_079/0018294/EC | European Regional Development Fund (Europski Fond za Regionalni Razvoj)
- Z.02.1.01/0.0/0.0/16_019/0000754/EC | European Regional Development Fund (Europski Fond za Regionalni Razvoj)
- 19-27454X/Grantová Agentura České Republiky (Grant Agency of the Czech Republic)
LinkOut - more resources
Full Text Sources

