In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial
- PMID: 35347281
- PMCID: PMC9018416
- DOI: 10.1038/s41591-022-01737-y
In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial
Abstract
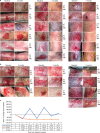
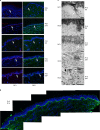
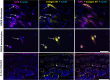
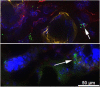
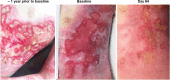
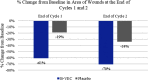
Recessive dystrophic epidermolysis bullosa (RDEB) is a lifelong genodermatosis associated with blistering, wounding, and scarring caused by mutations in COL7A1, the gene encoding the anchoring fibril component, collagen VII (C7). Here, we evaluated beremagene geperpavec (B-VEC), an engineered, non-replicating COL7A1 containing herpes simplex virus type 1 (HSV-1) vector, to treat RDEB skin. B-VEC restored C7 expression in RDEB keratinocytes, fibroblasts, RDEB mice and human RDEB xenografts. Subsequently, a randomized, placebo-controlled, phase 1 and 2 clinical trial (NCT03536143) evaluated matched wounds from nine RDEB patients receiving topical B-VEC or placebo repeatedly over 12 weeks. No grade 2 or above B-VEC-related adverse events or vector shedding or tissue-bound skin immunoreactants were noted. HSV-1 and C7 antibodies sometimes presented at baseline or increased after B-VEC treatment without an apparent impact on safety or efficacy. Primary and secondary objectives of C7 expression, anchoring fibril assembly, wound surface area reduction, duration of wound closure, and time to wound closure following B-VEC treatment were met. A patient-reported pain-severity secondary outcome was not assessed given the small proportion of wounds treated. A global assessment secondary endpoint was not pursued due to redundancy with regard to other endpoints. These studies show that B-VEC is an easily administered, safely tolerated, topical molecular corrective therapy promoting wound healing in patients with RDEB.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
M.P.M. received funding from Krystal Biotech to conduct this study through a sponsored research award administered through the Stanford University Office of Research Management. M.P.M. is also an investigator for the following companies that are studying molecular corrective therapies for recessive dystrophic epidermolysis bullosa: Castle Creek Pharmaceuticals, Abeona Therapeutics, WINGS therapeutics and Phoenix Tissue Repair. A.P.S. owns stock in Krystal Biotech. P.A., P.P.Z. and S.O., as well as H.L., N.R., N.S., M.O. and S.M.K. are employees of Krystal Biotech. All other authors have no competing interests.
Figures


Comment in
-
Restoring type VII collagen in skin.Med. 2022 May 13;3(5):273-275. doi: 10.1016/j.medj.2022.04.008. Epub 2022 May 13. Med. 2022. PMID: 35584643
-
The joint battle to tackle epidermolysis bullosa through gene therapy.Trends Mol Med. 2022 Jul;28(7):533-535. doi: 10.1016/j.molmed.2022.05.001. Epub 2022 May 19. Trends Mol Med. 2022. PMID: 35599142
References
-
- Marinkovich, M. P. Inherited epidermolysis bullosa. In Fitzpatrick’s Dermatology 9th edn (eds Kang, S. et al.) Ch. 60 (McGraw Hill Medical, 2019).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

