The role of artificial intelligence in paediatric neuroradiology
- PMID: 35347371
- PMCID: PMC9537195
- DOI: 10.1007/s00247-022-05322-w
The role of artificial intelligence in paediatric neuroradiology
Abstract
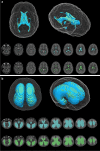
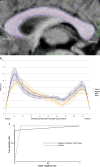
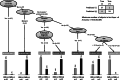
Imaging plays a fundamental role in the managing childhood neurologic, neurosurgical and neuro-oncological disease. Employing multi-parametric MRI techniques, such as spectroscopy and diffusion- and perfusion-weighted imaging, to the radiophenotyping of neuroradiologic conditions is becoming increasingly prevalent, particularly with radiogenomic analyses correlating imaging characteristics with molecular biomarkers of disease. However, integration into routine clinical practice remains elusive. With modern multi-parametric MRI now providing additional data beyond anatomy, informing on histology, biology and physiology, such metric-rich information can present as information overload to the treating radiologist and, as such, information relevant to an individual case can become lost. Artificial intelligence techniques are capable of modelling the vast radiologic, biological and clinical datasets that accompany childhood neurologic disease, such that this information can become incorporated in upfront prognostic modelling systems, with artificial intelligence techniques providing a plausible approach to this solution. This review examines machine learning approaches than can be used to underpin such artificial intelligence applications, with exemplars for each machine learning approach from the world literature. Then, within the specific use case of paediatric neuro-oncology, we examine the potential future contribution for such artificial intelligence machine learning techniques to offer solutions for patient care in the form of decision support systems, potentially enabling personalised medicine within this domain of paediatric radiologic practice.
Keywords: Artificial intelligence; Children; Machine learning; Magnetic resonance imaging; Neuroradiology; Radiogenomics.
© 2022. Crown.
Conflict of interest statement
None
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

