AAV-Mediated Artificial miRNA Reduces Pathogenic Polyglucosan Bodies and Neuroinflammation in Adult Polyglucosan Body and Lafora Disease Mouse Models
- PMID: 35347645
- PMCID: PMC9294094
- DOI: 10.1007/s13311-022-01218-7
AAV-Mediated Artificial miRNA Reduces Pathogenic Polyglucosan Bodies and Neuroinflammation in Adult Polyglucosan Body and Lafora Disease Mouse Models
Abstract
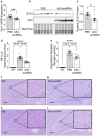
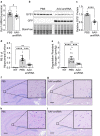
Adult polyglucosan body disease (APBD) and Lafora disease (LD) are autosomal recessive glycogen storage neurological disorders. APBD is caused by mutations in the glycogen branching enzyme (GBE1) gene and is characterized by progressive upper and lower motor neuron dysfunction and premature death. LD is a fatal progressive myoclonus epilepsy caused by loss of function mutations in the EPM2A or EPM2B gene. These clinically distinct neurogenetic diseases share a common pathology. This consists of time-dependent formation, precipitation, and accumulation of an abnormal form of glycogen (polyglucosan) into gradually enlarging inclusions, polyglucosan bodies (PBs) in ever-increasing numbers of neurons and astrocytes. The growth and spread of PBs are followed by astrogliosis, microgliosis, and neurodegeneration. The key defect in polyglucosans is that their glucan branches are longer than those of normal glycogen, which prevents them from remaining in solution. Since the lengths of glycogen branches are determined by the enzyme glycogen synthase, we hypothesized that downregulating this enzyme could prevent or hinder the generation of the pathogenic PBs. Here, we pursued an adeno-associated virus vector (AAV) mediated RNA-interference (RNAi) strategy. This approach resulted in approximately 15% reduction of glycogen synthase mRNA and an approximately 40% reduction of PBs across the brain in the APBD and both LD mouse models. This was accompanied by improvements in early neuroinflammatory markers of disease. This work represents proof of principle toward developing a single lifetime dose therapy for two fatal neurological diseases: APBD and LD. The approach is likely applicable to other severe and common diseases of glycogen storage.
Keywords: AAV9; APBD; EPM2A; EPM2B; GBE1; GYS1; RNAi; miRNA.
© 2022. The Author(s).
Figures



Comment in
-
Two Diseases-One Preclinical Treatment Targeting Glycogen Synthesis.Neurotherapeutics. 2022 Apr;19(3):977-981. doi: 10.1007/s13311-022-01240-9. Epub 2022 Apr 22. Neurotherapeutics. 2022. PMID: 35460010 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

