Platelet-Derived Growth Factor Receptor Type α Activation Drives Pulmonary Vascular Remodeling Via Progenitor Cell Proliferation and Induces Pulmonary Hypertension
- PMID: 35348002
- PMCID: PMC9075467
- DOI: 10.1161/JAHA.121.023021
Platelet-Derived Growth Factor Receptor Type α Activation Drives Pulmonary Vascular Remodeling Via Progenitor Cell Proliferation and Induces Pulmonary Hypertension
Abstract
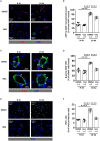
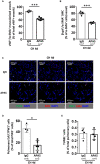
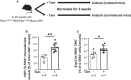
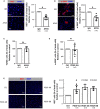
Background Platelet-derived growth factor is a major regulator of the vascular remodeling associated with pulmonary arterial hypertension. We previously showed that protein widely 1 (PW1+) vascular progenitor cells participate in early vessel neomuscularization during experimental pulmonary hypertension (PH) and we addressed the role of the platelet-derived growth factor receptor type α (PDGFRα) pathway in progenitor cell-dependent vascular remodeling and in PH development. Methods and Results Remodeled pulmonary arteries from patients with idiopathic pulmonary arterial hypertension showed an increased number of perivascular and vascular PW1+ cells expressing PDGFRα. PW1nLacZ reporter mice were used to follow the fate of pulmonary PW1+ progenitor cells in a model of chronic hypoxia-induced PH development. Under chronic hypoxia, PDGFRα inhibition prevented the increase in PW1+ progenitor cell proliferation and differentiation into vascular smooth muscle cells and reduced pulmonary vessel neomuscularization, but did not prevent an increased right ventricular systolic pressure or the development of right ventricular hypertrophy. Conversely, constitutive PDGFRα activation led to neomuscularization via PW1+ progenitor cell differentiation into new smooth muscle cells and to PH development in male mice without fibrosis. In vitro, PW1+ progenitor cell proliferation, but not differentiation, was dependent on PDGFRα activity. Conclusions These results demonstrate a major role of PDGFRα signaling in progenitor cell-dependent lung vessel neomuscularization and vascular remodeling contributing to PH development, including in idiopathic pulmonary arterial hypertension patients. Our findings suggest that PDGFRα blockers may offer a therapeutic add-on strategy to combine with current pulmonary arterial hypertension treatments to reduce vascular remodeling. Furthermore, our study highlights constitutive PDGFRα activation as a novel experimental PH model.
Keywords: fibrosis; platelet‐derived growth factor receptor alpha; pulmonary hypertension; stem cells; vascular remodeling.
Figures



References
-
- Ricard N, Tu LY, Le Hiress M, Huertas A, Phan C, Thuillet R, Sattler C, Fadel E, Seferian A, Montani D, et al. Increased pericyte coverage mediated by endothelial‐derived fibroblast growth factor‐2 and interleukin‐6 is a source of smooth muscle‐like cells in pulmonary hypertension. Circulation. 2014;129:1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469 - DOI - PubMed
-
- Dierick F, Héry T, Hoareau‐Coudert B, Mougenot N, Monceau V, Claude C, Crisan M, Besson V, Dorfmüller P, Marodon G, et al. Resident PW1+ progenitor cells participate in vascular remodeling during pulmonary arterial hypertension. Circ Res. 2016;118:822–833. doi: 10.1161/circresaha.115.307035 - DOI - PubMed
-
- Bordenave J, Tu LY, Berrebeh N, Thuillet R, Cumont A, Le Vely B, Fadel E, Nadaud S, Savale L, Humbert M, et al. Lineage tracing reveals the dynamic contribution of pericytes to the blood vessel remodeling in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2020;40:766–782. doi: 10.1161/ATVBAHA.119.313715 - DOI - PubMed
-
- Perros F, Montani D, Dorfmüller P, Durand‐Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, et al. Platelet‐derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:81–88. doi: 10.1164/rccm.200707-1037OC - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

