Modeling type 2 diabetes in rats by administering tacrolimus
- PMID: 35348048
- PMCID: PMC8966987
- DOI: 10.1080/19382014.2022.2051991
Modeling type 2 diabetes in rats by administering tacrolimus
Abstract
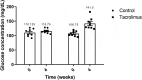
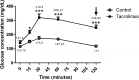
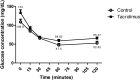
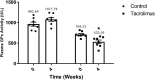
The prevalence of diabetes is rapidly increasing. The current number of diagnosed cases is ~422 million, expected to reach ~640 million by 2040. Type 2 diabetes, which constitutes ~95% of the cases, is characterized by insulin resistance and a progressive loss of β-cell function. Despite intense research efforts, no treatments are yet able to cure the disease or halt its progression. Since all existing animal models of type 2 diabetes have serious drawbacks, one is needed that represents the complete pathogenesis, is low cost and non-obese, and can be developed relatively quickly. The aim of this study was to evaluate a low-cost, non-obese model of type 2 diabetes engendered by administering a daily high dose of tacrolimus (an immunosuppressant) to Wistar rats for 4 weeks. The biochemical and antioxidant markers were measured at basal and after the 4-week tacrolimus treatment. At week 4, the values of these parameters closely resembled those observed in human type 2 diabetes, including fasting blood glucose at 141.5 mg/dL, blood glucose greater than 200 mg/dL at 120 min of the glucose tolerance test, blood glucose at varied levels in the insulin tolerance test, and elevated levels of cholesterol and triglyceride. The tacrolimus treatment produced hypoinsulinemia and sustained hyperglycemia, probably explained by the alteration found in pancreatic β-cell function and morphology. This model should certainly be instrumental for evaluating possible type 2 diabetes treatments, and for designing new immunosuppressants that do not cause pancreatic damage, type 2 diabetes, or new-onset diabetes after transplantation (NODAT).
Keywords: Wistar rat; hyperglycemia; modeling type 2 diabetes; pancreatic damage; tacrolimus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







References
-
- World Health Organization . Global report in diabetes. Geneva: World Health Organ. 2016.
-
- World Health Organization . Noncommunicable diseases country profiles.Geneva. World Health Organ. 2018. License: CC BY-NC-SA 3.0 IGO.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
