Niclosamide-A promising treatment for COVID-19
- PMID: 35348204
- PMCID: PMC9111792
- DOI: 10.1111/bph.15843
Niclosamide-A promising treatment for COVID-19
Abstract
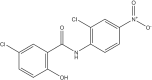
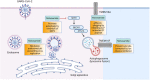
Vaccines have reduced the transmission and severity of COVID-19, but there remains a paucity of efficacious treatment for drug-resistant strains and more susceptible individuals, particularly those who mount a suboptimal vaccine response, either due to underlying health conditions or concomitant therapies. Repurposing existing drugs is a timely, safe and scientifically robust method for treating pandemics, such as COVID-19. Here, we review the pharmacology and scientific rationale for repurposing niclosamide, an anti-helminth already in human use as a treatment for COVID-19. In addition, its potent antiviral activity, niclosamide has shown pleiotropic anti-inflammatory, antibacterial, bronchodilatory and anticancer effects in numerous preclinical and early clinical studies. The advantages and rationale for nebulized and intranasal formulations of niclosamide, which target the site of the primary infection in COVID-19, are reviewed. Finally, we give an overview of ongoing clinical trials investigating niclosamide as a promising candidate against SARS-CoV-2.
Keywords: COVID-19; clinical trials; niclosamide; repurposing.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
JC and RS acknowledge institutional grants from Union Therapeutics for the conduct of investigator initiated clinical trials of niclosamide. MS is a shareholder of UNION Therapeutics, and AW benefits from an employee incentive scheme. The other authors declare no conflict of interest.
Figures

References
-
- Abdulamir, A. S. , Gorial, F. I. , Saadi, S. J. , Maulood, M. F. , Hashim, H. A. , Alnuaimi, A. S. , & Abdulrrazaq, M. K. (2021). A randomised controlled trial of effectiveness and safety of Niclosamide as add on therapy to the standard of care measures in COVID‐19 management. Annals of Medicine and Surgery, 69, 102779. 10.1016/j.amsu.2021.102779 - DOI - PMC - PubMed
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Beuve, A. , Brouckaert, P. , Bryant, C. , Burnett, J. C. , Farndale, R. W. , Friebe, A. , Garthwaite, J. , … Waldman, S. A. (2021a). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(S1), S264–S312. 10.1111/bph.15541 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021b). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

