Evaluation of correlated studies using liquid cell and cryo-transmission electron microscopy: Hydration of calcium sulphate and the phase transformation pathways of bassanite to gypsum
- PMID: 35348205
- PMCID: PMC10084335
- DOI: 10.1111/jmi.13102
Evaluation of correlated studies using liquid cell and cryo-transmission electron microscopy: Hydration of calcium sulphate and the phase transformation pathways of bassanite to gypsum
Abstract
Insight into the nucleation, growth and phase transformations of calcium sulphate could improve the performance of construction materials, reduce scaling in industrial processes and aid understanding of its formation in the natural environment. Recent studies have suggested that the calcium sulphate pseudo polymorph, gypsum (CaSO4 ·2H2 O) can form in aqueous solution via a bassanite (CaSO4 ·0.5H2 O) intermediate. Some in situ experimental work has also suggested that the transformation of bassanite to gypsum can occur through an oriented assembly mechanism. In this work, we have exploited liquid cell transmission electron microscopy (LCTEM) to study the transformation of bassanite to gypsum in an undersaturated aqueous solution of calcium sulphate. This was benchmarked against cryogenic TEM (cryo-TEM) studies to validate internally the data obtained from the two microscopy techniques. When coupled with Raman spectroscopy, the real-time data generated by LCTEM, and structural data obtained from cryo-TEM show that bassanite can transform to gypsum via more than one pathway, the predominant one being dissolution/reprecipitation. Comparisons between LCTEM and cryo-TEM also show that the transformation is slower within the confined region of the liquid cell as compared to a bulk solution. This work highlights the important role of a correlated microscopy approach for the study of dynamic processes such as crystallisation from solution if we are to extract true mechanistic understanding.
Keywords: calcium sulphate; cryogenic TEM; crystallisation; liquid phase TEM.
© 2022 The Authors. Journal of Microscopy published by John Wiley & Sons Ltd on behalf of Royal Microscopical Society.
Figures
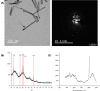




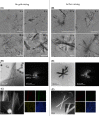
References
-
- Jin, B. , Liu, Z. , & Tang, R. (2020). Recent experimental explorations of non‐classical nucleation. CrystEngComm, 22(24), 4057–4073.
-
- Cookman, J. , Hamilton, V. , Price, L. S. , Hall, S. R. , & Bangert, U. (2020). Visualising early‐stage liquid phase organic crystal growth via liquid cell electron microscopy. Nanoscale, 12(7), 4636–4644. - PubMed
-
- Nielsen, M. H. , Aloni, S. , & De Yoreo, J. J. (2014a). In situ TEM imaging of CaCO3nucleation reveals coexistence of direct and indirect pathways. Science, 345(6201), 1158–1162. - PubMed
-
- Nielsen, M. H. , Li, D. , Zhang, H. , Aloni, S. , Han, T. Y.‐J. , Frandsen, C. , Seto, J. , Banfield, J. F. , Cölfen, H. , & De Yoreo, J. J. (2014b). Investigating processes of nanocrystal formation and transformation via liquid cell TEM. Microscopy and Microanalysis, 20(2), 425–436. - PubMed
-
- de Jonge, N. , & Ross, F. M. (2011). Electron microscopy of specimens in liquid. Nature Nanotechnology, 6(11), 695–704. - PubMed

