A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings
- PMID: 35348391
- PMCID: PMC10563532
- DOI: 10.1177/19322968221085273
A Glycemia Risk Index (GRI) of Hypoglycemia and Hyperglycemia for Continuous Glucose Monitoring Validated by Clinician Ratings
Abstract
Background: A composite metric for the quality of glycemia from continuous glucose monitor (CGM) tracings could be useful for assisting with basic clinical interpretation of CGM data.
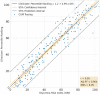
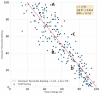
Methods: We assembled a data set of 14-day CGM tracings from 225 insulin-treated adults with diabetes. Using a balanced incomplete block design, 330 clinicians who were highly experienced with CGM analysis and interpretation ranked the CGM tracings from best to worst quality of glycemia. We used principal component analysis and multiple regressions to develop a model to predict the clinician ranking based on seven standard metrics in an Ambulatory Glucose Profile: very low-glucose and low-glucose hypoglycemia; very high-glucose and high-glucose hyperglycemia; time in range; mean glucose; and coefficient of variation.
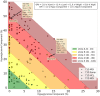
Results: The analysis showed that clinician rankings depend on two components, one related to hypoglycemia that gives more weight to very low-glucose than to low-glucose and the other related to hyperglycemia that likewise gives greater weight to very high-glucose than to high-glucose. These two components should be calculated and displayed separately, but they can also be combined into a single Glycemia Risk Index (GRI) that corresponds closely to the clinician rankings of the overall quality of glycemia (r = 0.95). The GRI can be displayed graphically on a GRI Grid with the hypoglycemia component on the horizontal axis and the hyperglycemia component on the vertical axis. Diagonal lines divide the graph into five zones (quintiles) corresponding to the best (0th to 20th percentile) to worst (81st to 100th percentile) overall quality of glycemia. The GRI Grid enables users to track sequential changes within an individual over time and compare groups of individuals.
Conclusion: The GRI is a single-number summary of the quality of glycemia. Its hypoglycemia and hyperglycemia components provide actionable scores and a graphical display (the GRI Grid) that can be used by clinicians and researchers to determine the glycemic effects of prescribed and investigational treatments.
Keywords: ambulatory glucose profile; composite metric; continuous glucose monitor; diabetes; glycemia risk index; hyperglycemia; hypoglycemia; time in range.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.C.K. is a consultant to AI Health, Dexcom, Eli Lilly, EOFlow, Integrity, Lifecare, Medtronic, Novo, Roche Diagnostics, Rockley Photonics, and Thirdwayv. D.R. is a consultant to Eli Lilly & Co. Inc. and Better Therapeutics, Inc. He has previously provided consulting services to multiple companies and organizations that produce glucose meters, continuous glucose monitors, insulin pumps, closed-loop systems, software applications, clinical decision support systems, and other technologies for management of patients with diabetes. D.K. has received remuneration for participation in Advisory Boards from Sanofi, Novo Nordisk, and Abbott Diabetes Care. He also has received research support from Novo Nordisk and Abbott Diabetes Care and has financial interests in Glooko, Hi.Health and SNAQ. DA has received speaker’s honoraria from Lilly Diabetes, Xeris Pharmaceuticals, and Zealand Pharma. He has received consulting fees from Ascensia Diabetes Care, Roche Diagnostics, and Senseonics. A.L.P. reports participation on an advisory board for Abbott Diabetes Care, Astra Zeneca, Eli Lilly, Medscape, Novo Nordisk, Vertex, and Zealand Research. She reports support from Dexcom and Insulet and receives donated devices from Abbott Diabetes Care. She has stock options with Omada Health and Teladoc. JJS has received remuneration for participation in one Advisory Board meeting for Dexcom. G.E.U. reports research funds to Emory University from Astra Zeneca and Dexcom. NYX is a consultant to Abbott Diabetes Care. K.T.N. is a consultant to Abbott Diabetes Care. G.S. reports educational grants from Abbott Diabetes Care and Sanofi. He also reports research grants from Abbott Diabetes Care. T.S.B. reports research support from Abbott Diabetes, Abbott Rapid Diagnostics, Biolinq, Capillary Biomedical, Dexcom, Eli Lilly, Kowa, Livongo, MannKind, Medtronic, Novo Nordisk, REMD, Sanofi, Sanvita, Senseonics, Viacyte, vTv Therapeutics, and Zealand Pharma. He also reports consulting honoraria from Abbott, CeQur, Lifescan, MannKind, Medtronic, Novo, and Sanofi, as well as speaking Honoraria from BD, Medtronic, Sanofi. P.-Y.B. has received speaker honoraria from Abbott, Roche, Eli Lilly, Novo Nordisk, and Sanofi, and served on advisory board panels for Abbott, Diabeloop, Roche, Medtronic, Dexcom, Insulet, LifeScan, Eli Lilly, Novo Nordisk, and Sanofi. Th.B. reports research support from Lilly, Novo, Dexcom, Tandem, Abbott, and Viacyte. He reports research site research support from Medtronic. He is a speaker for Lilly, Novo, BI, AZ, and Zealand. J.R.C. has a financial interest in Pacific Diabetes Technologies Inc., a company that may have a commercial interest in the results of this type of research and technology. J.R.C. also reports advisory board participation for Zealand Pharma, Novo Nordisk, Insulet, and AstraZeneca, and her institution has received research funding from Dexcom. P.C. has received personal fees from Medtronic, Dexcom, Abbott, Glooko, Insulet, Sanofi, Lilly, and Novo Nordisk. He has received research support from Abbott, Dexcom, Medtronic, and Novo Nordisk. M.A.C. is the Chief Medical Officer at Glooko. He reports material grant support from Abbott Diabetes Care and Dexcom for investigator-initiated studies. T.D. has received speaker’s honoraria and research support from and has consulted for Abbott, AstraZeneca, Boehringer, Dexcom, Lilly, Medtronic, Novo Nordisk, Roche, Sanofi, and Ypsomed and is a shareholder of DreaMed Ltd. F.J.D. reports equity, licensed IP, and is a member of the Scientific Advisory Board of Mode AGC. K.M.D. reports research support from Dexcom, Abbott, Sanofi, and Viacyte. She has consulted for Dexcom, Eli Lilly, and Boehringer Ingelheim. She reports honoraria from Elsevier, Medscape, Academy for Continued Healthcare Learning, Cardiometabolic Health Conference, UptoDate. S.V.E. is a medical advisor for Lilly, Sanofi, AZ, Brightsight, and BOD Senseonics. J.C.E. is a paid consultant for AI Health. AI Health played no role in the design, execution, analysis, or write up of this work. AI Health did not play a role in the decision to publish this manuscript and had no editorial input. J.C.E. receives grant support from the Food and Drug Administration under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics, and by grants UL1TR001855 and UL1TR000130 from the National Center for Advancing Translational Science (NCATS) of the US National Institutes of Health. The funding sources had no involvement in the development of this manuscript or in the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the FDA or the NIH. G.A.F. has disclosures with 180 LifeScience, Abvance, Adocia, American Federation for Aging Research, Aerami, ALMS, Amolyt, Baker Heart & Diabetes (formerly Cardiora), Berg, Biocon, CarThera, Checkpoint, Diamyd, Diasome, Entera Bio, Enterin, Fortress, Glyscend, Hagar, IM Therapeutics, i2O, Innoneo, Mars Symbioscience, Linear, MMD, Northwestern University, NuSirt, Oberland, Oramed, Orgenesis, Pano, RenovoRx, Rivus, SFC Fluidics, Stalicla, Surf Bio, TixiMED, Unify, Veroscience, Verthermia, Zucara. GPF reports research support from Medtronic, Dexcom, Insulet, Tandem, Abbott, Lilly, and Beta Bionics. He reports speaking/consulting/advisory board fees from Medtronic, Dexcom, Tandem, Insulet, Abbott, Lilly, and Beta Bionics. GF is general manager and medical director of the Institute for Diabetes Technology (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies, for example, with medical devices for diabetes therapy on its own initiative and on behalf of various companies. GF/IDT have received speakers’ honoraria or consulting fees from Abbott, Ascensia, Berlin Chemie, Beurer, BOYDsense, CRF Health, Dexcom, i-SENS, Lilly, Metronom, MySugr, Novo Nordisk, Pharmasens, Roche, Sanofi, Sensile, Terumo, and Ypsomed. R.J.G. received research support to Emory University for investigator-initiated studies from Novo Nordisk, Dexcom and Eli Lilly and consulting fees from Abbott Diabetes Care, Sanofi, Valeritas, Eli Lilly, Novo Nordisk, and Weight Watchers, outside of this work. R.J.G. is partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Numbers P30DK111024 (RJG), K23DK123384 (RJG). AMG has been a speaker for Sanofi, Medtronic, Abbott, Elli Lilly, and Novo Nordisk. L.H. is a consultant for a number of companies that are developing novel diagnostic and therapeutic options for diabetes treatment. He is a shareholder of the Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany. I.B.H. reports research support from Medtronic Diabetes, Insulet, and Beta Bionics. He has consulted for Abbott, Roche, Bigfoot, and GWave. T.D.H. is on the Advisory Board for Acella and Horizon therapeutics for which he receives no financial compensation. J.H.J. has received remuneration for participation in Advisory Boards from Sanofi, Novo Nordisk, Abbott Diabetes Care, Medtronic and Eli Lilly. He also has received research support from Novo Nordisk. SRJ has disclosures with Abbott, Novartis, Novo Nordisk, Zydus Cadila, Twinhealth, Marico, Franco Indian, USV, BI, AstraZeneca, Roche Diabetes Care, Lifescan, BayerZydus, and Glenmark. M.J. reports advisory fees, speaker fees and/or research funds from Abbott, Air Liquide Santé International, Amgen, Astrazeneca, Bayer, BMS, Boehringer Ingelheim, Dexcom, Glooko, Lifescan, Lilly, Medtronic, MSD, Nestle HomeCare, Novonordisk, Orkyn, Roche Diabetes, Sanofi, Vitalaire, and Voluntis. S.K.K. receives grant support from the UCSF Nutrition and Obesity Research Center (NIH P30 DK098722) and is an advisor to Yes Health, Suggestic, and Signos. R.A.L. is a consultant for Abbott Diabetes Care, Biolinq, Capillary Biomedical, Deep Valley Labs, Gluroo, Provention Bio, and Tidepool. M.C.L. reports research funding from Dexcom. L.A.L. has received research funding from, has acted as an adviser to, and/or has provided CME on behalf of Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Lexicon, Merck, Novo Nordisk, Pfizer, Sanofi, and Servier. M.L. has received research grants from Eli Lilly and Novo Nordisk and has been a consultant for Astra Zeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk. M.L.L. is an Advisory Board member for DiabetesWise PRO and dQ&A. J.K.M. is a member of the advisory board of Boehringer Ingelheim, Eli Lilly, Medtronic, Prediktor A/S, Roche Diabetes Care and Sanofi, and received speaker honoraria from Abbott Diabetes Care, AstraZeneca, Dexcom, Eli Lilly, Medtronic, Merk Sharp & Dohme, Novo Nordisk A/S, Roche Diabetes Care, Sanofi, Servier, and Takeda. UM reports research funding from Clementia Pharmaceutical and is an advisor for Ryse Health. J.M. is a full-time employee at Glytec. J.D.M. is a consultant/speaker for Medtronic Diabetes and an advisor for MannKind, Inc. He reports grant support from Dexcom, Inc. V.M. has acted as a consultant and speaker, received research or educational grants from Abbott, Astra Zeneca, Boehringer Ingelheim, Dr. Reddy’s Laboratories, Johnson & Johnson, LifeScan, Lilly, MSD, Novartis, Novo Nordisk, Roche Diabetes Care India Pvt. Ltd, Sanofi-Aventis, USV Private Limited, and from other Indian pharmaceutical companies. JHN is a consultant for Abbott, Dexcom, and Roche. He reports research support and speaker honorarium from Abbott, and speaker honorarium from Roche. KN received funding for her institution for participating in advisory boards from Medtronic, Convatec, and Novo Nordisk and for lecturing from Sanofi, Novo Nordisk, Medtronic, and Dexcom. Her institution received funding for studies she performed from Zealand Pharma, RSP Systems, Novo Nordisk, Medtronic and Dexcom. F.J.P. reports research support from Merck, Dexcom, and Insulet. He is a consultant for Boehringer Ingelheim, Dexcom, and AI Health. A.P.-T. reports that Scripps Health receives fees for advising services with no transfer to author. M.P. reports grants/research support to his Institute from Medtronic, Novo Nordisk, Roche, Eli Lilly, Sanofi, Pfizer, Insulet, Opko, Dexcom, DOMPE DreaMed–Diabetes, and NG Solutions. He reports honoraria or consultation fees from Sanofi, Medtronic, Novo Nordisk, Eli Lilly and Pfizer. He is on the Advisory Board for Sanofi, Medtronic, AstraZeneca, Eli Lilly, Insulet, Pfizer, and DOMPE. He is a stock shareholder in NG Solutions Ltd. And DreaMed-Diabetes Ltd. He is a consultant for Qulab Medical. W.H.P. is a consultant for Dexcom and Abbott Diabetes Care. S.J.R. is an inventor on patents related to the bionic pancreas technology that are assigned to Massachusetts General Hospital and licensed to Beta Bionics; has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia; serves on the scientific advisory boards of Unomedical and Companion Medical; has received consulting fees from Beta Bionics, Novo Nordisk, Senseonics, and Flexion Therapeutics; has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics; and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascensia, Senseonics, Adocia, and Tandem Diabetes. V.N.S.’s employer received grants from Sanofi, Dexcom, Insulet, Abbott, Novo Nordisk, and Eli Lilly. V.N.S. received honorarium/consulting fees through University of Colorado from Dexcom, Insulet, Sanofi, and Medscape. These disclosures are outside the submitted work. J.L.S. has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for Eli Lilly, Lexicon, Medtronic, and Sanofi. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex. K.S. is a full-time employee of University of North Carolina at Chapel Hill and has received grants from Arkray Inc., Dexcom Inc., Terumo Corp., and PTS Inc. E.K.S. reports that this work was supported in part by a VA MERIT award from the US Department of Veterans Affairs Clinical Sciences Research and Development Service (1I01CX001825). E.K.S. has received unrestricted research support from Dexcom (to the Baltimore VA Medical Center and to the University of Maryland) for the conduction of clinical trials. A.W. reports research support from Novo Nordisk, UnitedHealth Group, and Eli Lilly. E.E.W. is an advisor, consultant and speaker for Abbott Diabetes Care and Bayer, an advisor and speaker for Boehringer Ingelheim and Sanofi, a speaker for Eli Lilly, and an advisor Medtronic. B.K. reports research support handled by the University of Virginia from Dexcom, Novo Nordisk, Tandem Diabetes Care. He also reports patent royalties handled by the University of Virginia from Dexcom, Johnson & Johnson, Novo Nordisk, and Sanofi. He has received presentation honoraria from Tandem. J.W., M.K., C.L., D.L., M.S.D.A., M.E.A.-S., G.A.-P., A.B., Ta.B., S.Y.B., B.W.B., M.D.B., J.G.C., K.Y.C., K.L.C., C.B.C., A.D., N.E., H.A.G., R.H., L.J., L.L., K.M.M., B.M., N.N.M., A.M., D.N.N., T.P., R.P.-B., G.R., E.-J.R., D.W., K.W., M.E.W., H.W., and M.Z. have nothing relevant to disclose.
Figures




References
-
- Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2021;44(11):2589-2625. - PubMed
-
- Giglio RV, Stoian AP, Haluzik M, et al. Novel molecular markers of cardiovascular disease risk in type 2 diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2021;1867(8):166148. - PubMed
-
- American Diabetes Association Professional Practice Committee. 6. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S83-S96. - PubMed
-
- AGP Report. Welcome to AGP report. Date unknown. http://www.agpreport.org/agp/. Accessed December 5, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

