The bidirectional lung brain-axis of amyloid-β pathology: ozone dysregulates the peri-plaque microenvironment
- PMID: 35348636
- PMCID: PMC10169526
- DOI: 10.1093/brain/awac113
The bidirectional lung brain-axis of amyloid-β pathology: ozone dysregulates the peri-plaque microenvironment
Abstract
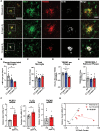
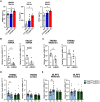
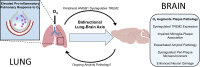
The mechanisms underlying how urban air pollution affects Alzheimer's disease (AD) are largely unknown. Ozone (O3) is a reactive gas component of air pollution linked to increased AD risk, but is confined to the respiratory tract after inhalation, implicating the peripheral immune response to air pollution in AD neuropathology. Here, we demonstrate that O3 exposure impaired the ability of microglia, the brain's parenchymal immune cells, to associate with and form a protective barrier around Aβ plaques, leading to augmented dystrophic neurites and increased Aβ plaque load. Spatial proteomic profiling analysis of peri-plaque proteins revealed a microenvironment-specific signature of dysregulated disease-associated microglia protein expression and increased pathogenic molecule levels with O3 exposure. Unexpectedly, 5xFAD mice exhibited an augmented pulmonary cell and humoral immune response to O3, supporting that ongoing neuropathology may regulate the peripheral O3 response. Circulating HMGB1 was one factor upregulated in only 5xFAD mice, and peripheral HMGB1 was separately shown to regulate brain Trem2 mRNA expression. These findings demonstrate a bidirectional lung-brain axis regulating the central and peripheral AD immune response and highlight this interaction as a potential novel therapeutic target in AD.
Keywords: HMGB1; TREM2; air pollution; amyloid plaque; lung-brain axis; microglia.
Published by Oxford University Press on behalf of the Guarantors of Brain 2022.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Jayaraj RL, Rodriguez EA, Wang Y, Block ML. Outdoor ambient air pollution and neurodegenerative diseases: The neuroinflammation hypothesis. Curr Environ Health Rep. 2017;4(2):166–179. - PubMed
-
- Croze ML, Zimmer L. Ozone atmospheric pollution and Alzheimer’s disease: From epidemiological facts to molecular mechanisms. J Alzheimer's Dis. 2018;62:503–522. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

