Tafasitamab mediates killing of B-cell non-Hodgkin's lymphoma in combination with γδ T cell or allogeneic NK cell therapy
- PMID: 35348812
- PMCID: PMC9519642
- DOI: 10.1007/s00262-022-03165-w
Tafasitamab mediates killing of B-cell non-Hodgkin's lymphoma in combination with γδ T cell or allogeneic NK cell therapy
Abstract
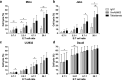
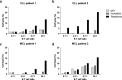
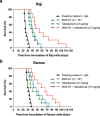
Tafasitamab is an Fc-modified monoclonal antibody that binds to CD19, a cell-surface antigen that is broadly expressed on various types of B-cell non-Hodgkin's lymphoma (NHL). Antibody-dependent cellular cytotoxicity (ADCC), a key mode of action of tafasitamab, is mediated through the binding of tafasitamab's Fc region to FcγRIIIa receptors on immune effector cells and results in antitumor activity. Despite the proven clinical activity of tafasitamab in combination with lenalidomide in the treatment of diffuse large B-cell lymphoma (DLBCL), a higher number of immune cells in cancer patients may improve the activity of tafasitamab. Here, we characterized two ex vivo-expanded FcγRIIIa receptor-expressing cell types-γδ T and MG4101 natural killer (NK) cells-as effector cells for tafasitamab in vitro, and found that in the presence of these cells tafasitamab was able to induce ADCC against a range of NHL cell lines and patient-derived cells. We also explored the concept of effector cell supplementation during tafasitamab treatment in vivo by coadministering MG4101 NK cells in Raji and Ramos xenograft models of NHL. Combination treatment of tafasitamab and allogeneic MG4101 NK cells in these models demonstrated a survival benefit compared with tafasitamab or MG4101 monotherapy (Raji: 1.7- to 1.9-fold increase in lifespan; Ramos: 2.0- to 4.1-fold increase in lifespan). In conclusion, adoptive cell transfer of ex vivo-expanded allogeneic NK or autologous γδ T cells in combination with tafasitamab treatment may potentially be a promising novel approach to increase the number of immune effector cells and enhance the antitumor effect of tafasitamab.
Keywords: ADCC; B-cell non-Hodgkin’s lymphoma; Immunotherapy; NK cells; Tafasitamab; γδ T cells.
© 2022. The Author(s).
Conflict of interest statement
MP, JS, RB, CH, SS, and JE are employees of MorphoSys AG, Planegg, Germany. SS owns shares of MorphoSys AG. GC LabCell and MorphoSys AG report a joint patent application.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

