Altered Retinal Dopamine Levels in a Melatonin-proficient Mouse Model of Form-deprivation Myopia
- PMID: 35349094
- PMCID: PMC9468212
- DOI: 10.1007/s12264-022-00842-9
Altered Retinal Dopamine Levels in a Melatonin-proficient Mouse Model of Form-deprivation Myopia
Abstract
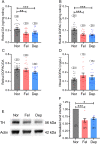
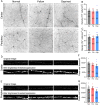
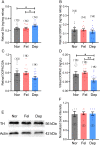
Reduced levels of retinal dopamine, a key regulator of eye development, are associated with experimental myopia in various species, but are not seen in the myopic eyes of C57BL/6 mice, which are deficient in melatonin, a neurohormone having extensive interactions with dopamine. Here, we examined the relationship between form-deprivation myopia (FDM) and retinal dopamine levels in melatonin-proficient CBA/CaJ mice. We found that these mice exhibited a myopic refractive shift in form-deprived eyes, which was accompanied by altered retinal dopamine levels. When melatonin receptors were pharmacologically blocked, FDM could still be induced, but its magnitude was reduced, and retinal dopamine levels were no longer altered in FDM animals, indicating that melatonin-related changes in retinal dopamine levels contribute to FDM. Thus, FDM is mediated by both dopamine level-independent and melatonin-related dopamine level-dependent mechanisms in CBA/CaJ mice. The previously reported unaltered retinal dopamine levels in myopic C57BL/6 mice may be attributed to melatonin deficiency.
Keywords: Dopamine; Melatonin; Mouse; Myopia; Refractive development; Retina.
© 2022. Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

