Cardiac pericyte reprogramming by MEK inhibition promotes arteriologenesis and angiogenesis of the ischemic heart
- PMID: 35349488
- PMCID: PMC9106362
- DOI: 10.1172/JCI152308
Cardiac pericyte reprogramming by MEK inhibition promotes arteriologenesis and angiogenesis of the ischemic heart
Abstract
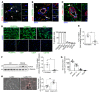
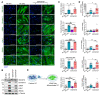
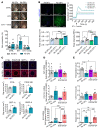
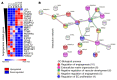
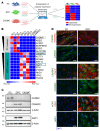
Pericytes (PCs) are abundant yet remain the most enigmatic and ill-defined cell population in the heart. Here, we investigated whether PCs can be reprogrammed to aid neovascularization. Primary PCs from human and mouse hearts acquired cytoskeletal proteins typical of vascular smooth muscle cells (VSMCs) upon exclusion of EGF/bFGF, which signal through ERK1/2, or upon exposure to the MEK inhibitor PD0325901. Differentiated PCs became more proangiogenic, more responsive to vasoactive agents, and insensitive to chemoattractants. RNA sequencing revealed transcripts marking the PD0325901-induced transition into proangiogenic, stationary VSMC-like cells, including the unique expression of 2 angiogenesis-related markers, aquaporin 1 (AQP1) and cellular retinoic acid-binding protein 2 (CRABP2), which were further verified at the protein level. This enabled us to trace PCs during in vivo studies. In mice, implantation of Matrigel plugs containing human PCs plus PD0325901 promoted the formation of αSMA+ neovessels compared with PC only. Two-week oral administration of PD0325901 to mice increased the heart arteriolar density, total vascular area, arteriole coverage by PDGFRβ+AQP1+CRABP2+ PCs, and myocardial perfusion. Short-duration PD0325901 treatment of mice after myocardial infarction enhanced the peri-infarct vascularization, reduced the scar, and improved systolic function. In conclusion, myocardial PCs have intrinsic plasticity that can be pharmacologically modulated to promote reparative vascularization of the ischemic heart.
Keywords: Angiogenesis; Cardiovascular disease; Microcirculation; Molecular biology; Vascular Biology.
Conflict of interest statement
Figures






Similar articles
-
Heart failure impairs the mechanotransduction properties of human cardiac pericytes.J Mol Cell Cardiol. 2021 Feb;151:15-30. doi: 10.1016/j.yjmcc.2020.10.016. Epub 2020 Nov 5. J Mol Cell Cardiol. 2021. PMID: 33159916
-
Inhibition of Ephrin-B2 in brain pericytes decreases cerebral pathological neovascularization in diabetic rats.PLoS One. 2019 Jan 8;14(1):e0210523. doi: 10.1371/journal.pone.0210523. eCollection 2019. PLoS One. 2019. PMID: 30620753 Free PMC article.
-
Role of TPBG (Trophoblast Glycoprotein) Antigen in Human Pericyte Migratory and Angiogenic Activity.Arterioscler Thromb Vasc Biol. 2019 Jun;39(6):1113-1124. doi: 10.1161/ATVBAHA.119.312665. Arterioscler Thromb Vasc Biol. 2019. PMID: 31018661
-
The role of endothelial cell-pericyte interactions in vascularization and diseases.J Adv Res. 2025 Jan;67:269-288. doi: 10.1016/j.jare.2024.01.016. Epub 2024 Jan 20. J Adv Res. 2025. PMID: 38246244 Free PMC article. Review.
-
Discovering cardiac pericyte biology: From physiopathological mechanisms to potential therapeutic applications in ischemic heart disease.Vascul Pharmacol. 2016 Nov;86:53-63. doi: 10.1016/j.vph.2016.05.009. Epub 2016 Jun 5. Vascul Pharmacol. 2016. PMID: 27268036 Review.
Cited by
-
Pericytes in the disease spotlight.Trends Cell Biol. 2024 Jan;34(1):58-71. doi: 10.1016/j.tcb.2023.06.001. Epub 2023 Jul 18. Trends Cell Biol. 2024. PMID: 37474376 Free PMC article. Review.
-
Approaches for the isolation and long-term expansion of pericytes from human and animal tissues.Front Cardiovasc Med. 2023 Jan 10;9:1095141. doi: 10.3389/fcvm.2022.1095141. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36704463 Free PMC article.
-
The longevity-associated BPIFB4 gene supports cardiac function and vascularization in ageing cardiomyopathy.Cardiovasc Res. 2023 Jul 4;119(7):1583-1595. doi: 10.1093/cvr/cvad008. Cardiovasc Res. 2023. PMID: 36635236 Free PMC article.
-
Biology and function of pericytes in the vascular microcirculation.Animal Model Exp Med. 2023 Aug;6(4):337-345. doi: 10.1002/ame2.12334. Epub 2023 Jun 14. Animal Model Exp Med. 2023. PMID: 37317664 Free PMC article. Review.
-
Generation and characterisation of scalable and stable human pluripotent stem cell-derived microvascular-like endothelial cells for cardiac applications.Angiogenesis. 2024 Aug;27(3):561-582. doi: 10.1007/s10456-024-09929-5. Epub 2024 May 22. Angiogenesis. 2024. PMID: 38775849 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

