Human TDP1, APE1 and TREX1 repair 3'-DNA-peptide/protein cross-links arising from abasic sites in vitro
- PMID: 35349719
- PMCID: PMC9023300
- DOI: 10.1093/nar/gkac185
Human TDP1, APE1 and TREX1 repair 3'-DNA-peptide/protein cross-links arising from abasic sites in vitro
Abstract
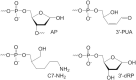
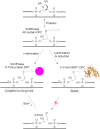
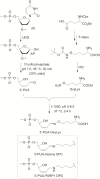
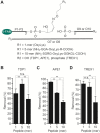
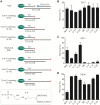
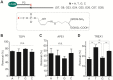
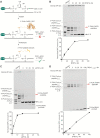
Histones and many other proteins react with abundant endogenous DNA lesions, apurinic/apyrimidinic (abasic, AP) sites and/or 3'-phospho-α,β-unsaturated aldehyde (3'-PUA), to form unstable but long-lived Schiff base DNA-protein cross-links at 3'-DNA termini (3'-PUA-protein DPCs). Poly (ADP-ribose) polymerase 1 (PARP1) cross-links to the AP site in a similar manner but the Schiff base is reduced by PARP1's intrinsic redox capacity, yielding a stable 3'-PUA-PARP1 DPC. Eradicating these DPCs is critical for maintaining the genome integrity because 3'-hydroxyl is required for DNA synthesis and ligation. But how they are repaired is not well understood. Herein, we chemically synthesized 3'-PUA-aminooxylysine-peptide adducts that closely resemble the proteolytic 3'-PUA-protein DPCs, and found that they can be repaired by human tyrosyl-DNA phosphodiesterase 1 (TDP1), AP endonuclease 1 (APE1) and three-prime repair exonuclease 1 (TREX1). We characterized these novel repair pathways by measuring the kinetic constants and determining the effect of cross-linked peptide length, flanking DNA structure, and the opposite nucleobase. We further found that these nucleases can directly repair 3'-PUA-histone DPCs, but not 3'-PUA-PARP1 DPCs unless proteolysis occurs initially. Collectively, we demonstrated that in vitro 3'-PUA-protein DPCs can be repaired by TDP1, APE1, and TREX1 following proteolysis, but the proteolysis is not absolutely required for smaller DPCs.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
Saccharomyces cerevisiae apurinic/apyrimidinic endonuclease 1 repairs abasic site-mediated DNA-peptide/protein cross-links.DNA Repair (Amst). 2023 Jun;126:103501. doi: 10.1016/j.dnarep.2023.103501. Epub 2023 Apr 8. DNA Repair (Amst). 2023. PMID: 37075541
-
Apurinic/apyrimidinic endonuclease 1 has major impact in prevention of suicidal covalent DNA-protein crosslink with apurinic/apyrimidinic site in cellular extracts.IUBMB Life. 2024 Nov;76(11):987-996. doi: 10.1002/iub.2890. Epub 2024 Jul 4. IUBMB Life. 2024. PMID: 38963041
-
In vitro eradication of abasic site-mediated DNA-peptide/protein cross-links by Escherichia coli long-patch base excision repair.J Biol Chem. 2022 Jul;298(7):102055. doi: 10.1016/j.jbc.2022.102055. Epub 2022 May 20. J Biol Chem. 2022. PMID: 35605665 Free PMC article.
-
Functions of the major abasic endonuclease (APE1) in cell viability and genotoxin resistance.Mutagenesis. 2020 Feb 13;35(1):27-38. doi: 10.1093/mutage/gez046. Mutagenesis. 2020. PMID: 31816044 Free PMC article. Review.
-
When DNA repair goes wrong: BER-generated DNA-protein crosslinks to oxidative lesions.DNA Repair (Amst). 2016 Aug;44:103-109. doi: 10.1016/j.dnarep.2016.05.014. Epub 2016 May 20. DNA Repair (Amst). 2016. PMID: 27264558 Free PMC article. Review.
Cited by
-
Key Amino Acid Residues of Mitochondrial Transcription Factor A Synergize with Abasic (AP) Site Dynamics To Facilitate AP-Lyase Reactions.ACS Chem Biol. 2023 May 19;18(5):1168-1179. doi: 10.1021/acschembio.3c00047. Epub 2023 Mar 17. ACS Chem Biol. 2023. PMID: 36930463 Free PMC article.
-
DNA-protein cross-links between abasic DNA damage and mitochondrial transcription factor A (TFAM).Nucleic Acids Res. 2023 Jan 11;51(1):41-53. doi: 10.1093/nar/gkac1214. Nucleic Acids Res. 2023. PMID: 36583367 Free PMC article.
-
DNA-Protein Cross-Links Derived from Abasic DNA Lesions: Recent Progress and Future Directions.Chem Res Toxicol. 2025 Jun 16;38(6):997-1005. doi: 10.1021/acs.chemrestox.5c00125. Epub 2025 May 19. Chem Res Toxicol. 2025. PMID: 40387817 Review.
-
ADP-ribosylation of NuMA promotes DNA single-strand break repair and transcription.Cell Rep. 2025 Jun 24;44(6):115737. doi: 10.1016/j.celrep.2025.115737. Epub 2025 May 20. Cell Rep. 2025. PMID: 40397572 Free PMC article.
-
Tyrosyl-DNA phosphodiesterase 1 (TDP1) and SPRTN protease repair histone 3 and topoisomerase 1 DNA-protein crosslinks in vivo.Open Biol. 2023 Oct;13(10):230113. doi: 10.1098/rsob.230113. Epub 2023 Oct 4. Open Biol. 2023. PMID: 37788708 Free PMC article.
References
-
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993; 362:709–715. - PubMed
-
- Higuchi K., Katayama T., Iwai S., Hidaka M., Horiuchi T., Maki H.. Fate of DNA replication fork encountering a single DNA lesion during oriC plasmid DNA replication in vitro. Genes Cells. 2003; 8:437–449. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

