Consensus subtypes of hepatocellular carcinoma associated with clinical outcomes and genomic phenotypes
- PMID: 35349735
- PMCID: PMC9519807
- DOI: 10.1002/hep.32490
Consensus subtypes of hepatocellular carcinoma associated with clinical outcomes and genomic phenotypes
Abstract
Background and aims: Although many studies revealed transcriptomic subtypes of HCC, concordance of the subtypes are not fully examined. We aim to examine a consensus of transcriptomic subtypes and correlate them with clinical outcomes.
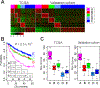
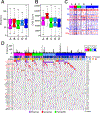
Approach and results: By integrating 16 previously established genomic signatures for HCC subtypes, we identified five clinically and molecularly distinct consensus subtypes. STM (STeM) is characterized by high stem cell features, vascular invasion, and poor prognosis. CIN (Chromosomal INstability) has moderate stem cell features, but high genomic instability and low immune activity. IMH (IMmune High) is characterized by high immune activity. BCM (Beta-Catenin with high Male predominance) is characterized by prominent β-catenin activation, low miRNA expression, hypomethylation, and high sensitivity to sorafenib. DLP (Differentiated and Low Proliferation) is differentiated with high hepatocyte nuclear factor 4A activity. We also developed and validated a robust predictor of consensus subtype with 100 genes and demonstrated that five subtypes were well conserved in patient-derived xenograft models and cell lines. By analyzing serum proteomic data from the same patients, we further identified potential serum biomarkers that can stratify patients into subtypes.
Conclusions: Five HCC subtypes are correlated with genomic phenotypes and clinical outcomes and highly conserved in preclinical models, providing a framework for selecting the most appropriate models for preclinical studies.
© 2022 American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflict of interests
The authors declare that they have no conflict of interests.
Figures





References
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–1255. - PubMed
-
- Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer 2015;15:653–667. - PubMed
-
- Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, et al. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology 2004;40:667–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

