Safety of ustekinumab in adolescent patients with moderate-to-severe plaque psoriasis: real-world evidence from an ongoing European study (NCT03218488)
- PMID: 35349743
- PMCID: PMC10286644
- DOI: 10.1111/jdv.18110
Safety of ustekinumab in adolescent patients with moderate-to-severe plaque psoriasis: real-world evidence from an ongoing European study (NCT03218488)
Conflict of interest statement
E. Mahé has undertaken paid activities as consultant, advisor or speaker for AbbVie, Amgen, Celgene, Janssen, Leo Pharma, Lilly and Novartis. A. Geldhof, M. Jazra, P. Bergmans and A. Azzabi are employees of Janssen and may own company stock/stock options. M.M.B. Seyger received grants from/was involved in clinical trials with AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Leo Pharma and Pfizer; and served as a consultant for AbbVie, Eli Lilly, Janssen, Leo Pharma, Novartis, Pfizer and UCB, with fees paid directly to her institution.
Figures
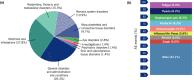
References
-
- Landells I, Marano C, Hsu M‐C et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate‐to‐severe plaque psoriasis: results of the randomized Phase 3 CADMUS study. J Am Acad Dermatol 2015; 73: 594–603. - PubMed
-
- Fortina AB, Bardazzi F, Berti S et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group. Eur J Pediatr 2017; 176: 1339–1354. - PubMed
-
- Phan C, Beauchet A, Burztejn A‐C et al. Biological treatments for paediatric psoriasis : a retrospective observational study on biological drug survival in daily practice in childhood psoriasis. J Eur Acad Dermatol Venereol 2019; 33: 1984–1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

