COVID-19 associated EBV reactivation and effects of ganciclovir treatment
- PMID: 35349757
- PMCID: PMC8959425
- DOI: 10.1002/iid3.597
COVID-19 associated EBV reactivation and effects of ganciclovir treatment
Abstract
Background: Systemic reactivation of Epstein-Barr virus (EBV) may occur in novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). However, the clinical consequences of EBV reactivation remain uncertain.
Methods: In this retrospective study, we screened 1314 patients with confirmed COVID-19 who died or were discharged between January 1, 2020 and March 12, 2020, in Wuhan Infectious Disease Hospital, Wuhan, China. Patients who had complete data for EBV serology and cytomegalovirus (CMV) serology were eligible. Serum levels of viral capsid antigen (VCA)-immunoglobulin G (IgG), Epstein-Barr nuclear antigen-IgG, VCA-IgM, early antigen (EA)-IgG, CMV-IgG, and CMV-IgM were compared between survivors and nonsurvivors. Dynamic changes of laboratory tests and outcomes were compared in patients with and without ganciclovir treatment. We used 1:1 matching based on age, gender, and illness severity to balance baseline characteristics.
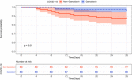
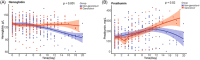
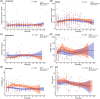
Results: EBV reactivation was present in 55 of 217 patients. EBV reactivation was associated with age (57.91 [13.19] vs. 50.28 [12.66] years, p < .001), female gender (31 [56%] vs. 60 [37%], p = .02). Patients with EBV reactivation have statistically nonsignificant higher mortality rate (12 [22%] vs. 18 [11%], p = .08). EA-IgG levels were significantly higher in nonsurvivors than in survivors (median difference: -0.00005, 95% confidence interval, CI [-3.10, 0.00], p = .05). As compared to patients with COVID-19 who did not receive ganciclovir therapy, ganciclovir-treated patients had improved survival rate (0.98, 95% CI [0.95, 1.00] vs. 0.88, 95% CI [0.81, 0.95], p = .01). Hemoglobin (p < .001) and prealbumin (p = .02) levels were significantly higher in ganciclovir-treated patients.
Conclusion: A high proportion of COVID-19 patients had EBV reactivation that may be associated with an increased risk of death. Whether treatment with ganciclovir may decrease the mortality of COVID-19 patients complicated with EBV reactivation warrants to be addressed in a placebo-controlled randomized trial in the future.
Keywords: COVID-19; EBV reactivation; ganciclovir; mortality.
© 2022 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Reactivation of EBV and CMV in Severe COVID-19-Epiphenomena or Trigger of Hyperinflammation in Need of Treatment? A Large Case Series of Critically ill Patients.J Intensive Care Med. 2022 Sep;37(9):1152-1158. doi: 10.1177/08850666211053990. Epub 2021 Nov 18. J Intensive Care Med. 2022. PMID: 34791940 Free PMC article.
-
Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients.Sci Rep. 2021 May 25;11(1):10902. doi: 10.1038/s41598-021-90351-y. Sci Rep. 2021. PMID: 34035353 Free PMC article.
-
[Analysis of CMV and EBV infection in healthy populations in China before and after the COVID-19 pandemic].Zhonghua Xue Ye Xue Za Zhi. 2024 Nov 14;45(11):986-990. doi: 10.3760/cma.j.cn121090-20240910-00342. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 39746690 Free PMC article. Chinese.
-
Serological Evidence for the Association Between Epstein-Barr Virus Infection and Sjögren's Syndrome.Front Immunol. 2020 Oct 30;11:590444. doi: 10.3389/fimmu.2020.590444. eCollection 2020. Front Immunol. 2020. PMID: 33193425 Free PMC article.
-
Use of rituximab in SARS-CoV-2-positive renal transplant recipient with EBV reactivation and probable haemophagocytic lymphohistiocytosis.CEN Case Rep. 2023 Feb;12(1):27-31. doi: 10.1007/s13730-022-00711-4. Epub 2022 Jun 22. CEN Case Rep. 2023. PMID: 35729310 Free PMC article. Review.
Cited by
-
Post-COVID cognitive dysfunction: current status and research recommendations for high risk population.Lancet Reg Health West Pac. 2023 Jul 5;38:100836. doi: 10.1016/j.lanwpc.2023.100836. eCollection 2023 Sep. Lancet Reg Health West Pac. 2023. PMID: 37457901 Free PMC article. Review.
-
Probable collagenous gastritis via Epstein-Barr virus reactivation in the setting of coronavirus disease 2019: a case report.J Med Case Rep. 2024 Dec 23;18(1):605. doi: 10.1186/s13256-024-04969-3. J Med Case Rep. 2024. PMID: 39710703 Free PMC article.
-
Anti-EBV: Artificial intelligence driven predictive modeling for repurposing drugs as potential antivirals against Epstein-Barr virus.Comput Struct Biotechnol J. 2025 May 1;27:1784-1799. doi: 10.1016/j.csbj.2025.04.042. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40458637 Free PMC article.
-
Epstein-Barr Virus and Human Herpesvirus-6 Reactivation in Acute COVID-19 Patients.Viruses. 2022 Aug 25;14(9):1872. doi: 10.3390/v14091872. Viruses. 2022. PMID: 36146679 Free PMC article.
-
Neurobiology of long-COVID: Hypotheses and unanswered questions.Anaesth Crit Care Pain Med. 2023 Jun;42(3):101201. doi: 10.1016/j.accpm.2023.101201. Epub 2023 Feb 15. Anaesth Crit Care Pain Med. 2023. PMID: 36801258 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

