The cellular slime mold Fonticula alba forms a dynamic, multicellular collective while feeding on bacteria
- PMID: 35349792
- PMCID: PMC9097593
- DOI: 10.1016/j.cub.2022.03.018
The cellular slime mold Fonticula alba forms a dynamic, multicellular collective while feeding on bacteria
Abstract
Multicellularity evolved in fungi and animals, or the opisthokonts, from their common amoeboflagellate ancestor but resulted in strikingly distinct cellular organizations. The origins of this multicellularity divergence are not known. The stark mechanistic differences that underlie the two groups and the lack of information about ancestral cellular organizations limits progress in this field. We discovered a new type of invasive multicellular behavior in Fonticula alba, a unique species in the opisthokont tree, which has a simple, bacteria-feeding sorocarpic amoeba lifestyle. This invasive multicellularity follows germination dependent on the bacterial culture state, after which amoebae coalesce to form dynamic collectives that invade virgin bacterial resources. This bacteria-dependent social behavior emerges from amoeba density and allows for rapid and directed invasion. The motile collectives have animal-like properties but also hyphal-like search and invasive behavior. These surprising findings enrich the diverse multicellularities present within the opisthokont lineage and offer a new perspective on fungal origins.
Keywords: amoeba; bacterial death phase; collective invasion; emerging models; evolutionary cell biology; multicellularity; opisthokonta; protist; slime mold.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



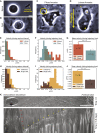
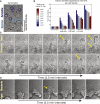
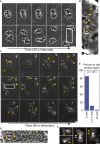
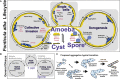
Comment in
-
Multicellularity: Amoebae follow the leader to food.Curr Biol. 2022 May 9;32(9):R418-R420. doi: 10.1016/j.cub.2022.03.067. Curr Biol. 2022. PMID: 35537392
References
-
- Niklas K.J., Newman S.A. The origins of multicellular organisms. Evol. Dev. 2013;15:41–52. - PubMed
-
- Rokas A. The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu. Rev. Genet. 2008;42:235–251. - PubMed
-
- Knoll A.H. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 2011;39:217–239.
-
- Nagy L.G., Kovács G.M., Krizsán K. Complex multicellularity in fungi: evolutionary convergence, single origin, or both? Biol. Rev. Camb. Philos. Soc. 2018;93:1778–1794. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

