T cell response against SARS-CoV-2 persists after one year in patients surviving severe COVID-19
- PMID: 35349827
- PMCID: PMC8957405
- DOI: 10.1016/j.ebiom.2022.103967
T cell response against SARS-CoV-2 persists after one year in patients surviving severe COVID-19
Abstract
Background: In critically ill COVID-19 patients, the initial response to SARS-CoV-2 infection is characterized by major immune dysfunctions. The capacity of these severe patients to mount a robust and persistent SARS-CoV-2 specific T cell response despite the presence of severe immune alterations during the ICU stay is unknown.
Methods: Critically ill COVID-19 patients were sampled five times during the ICU stay and 9 and 13 months afterwards. Immune monitoring included counts of lymphocyte subpopulations, HLA-DR expression on monocytes, plasma IL-6 and IL-10 concentrations, anti-SARS-CoV-2 IgG levels and T cell proliferation in response to three SARS-CoV-2 antigens.
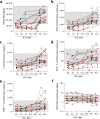
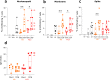
Findings: Despite the presence of major lymphopenia and decreased monocyte HLA-DR expression during the ICU stay, convalescent critically ill COVID-19 patients consistently generated adaptive and humoral immune responses against SARS-CoV-2 maintained for more than one year after hospital discharge. Patients with long hospital stays presented with stronger anti-SARS-CoV-2 specific T cell response but no difference in anti-SARS-CoV2 IgG levels.
Interpretation: Convalescent critically ill COVID-19 patients consistently generated a memory immune response against SARS-CoV-2 maintained for more than one year after hospital discharge. In recovered individuals, the intensity of SARS-CoV-2 specific T cell response was dependent on length of hospital stay.
Funding: This observational study was supported by funds from the Hospices Civils de Lyon, Fondation HCL, Claude Bernard Lyon 1 University and Région Auvergne Rhône-Alpes and by partial funding by REACTing (Research and ACTion targeting emerging infectious diseases) INSERM, France and a donation from Fondation AnBer (http://fondationanber.fr/).
Keywords: Critically ill patients; HLA-DR; Immune memory; SARS-CoV-2; Sepsis; T lymphocyte.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests MB, CT, KBG, VC and MAC are bioMérieux's employees. This private company had no role in the study design, result analysis and decision to publish this study. PV received consulting fees and payment for a literature review from Pfizer and Astellas. All other authors have declared no conflicts of interest.
Figures


References
-
- Gossez M., Malcus C., Demaret J., Frater J., Poitevin-Later F., Monneret G. Evaluation of a novel automated volumetric flow cytometer for absolute CD4+ T lymphocyte quantitation. Cytom B Clin Cytom. 2017;92(6):456–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

