Tocilizumab plus dexamethasone versus dexamethasone in patients with moderate-to-severe COVID-19 pneumonia: A randomised clinical trial from the CORIMUNO-19 study group
- PMID: 35350097
- PMCID: PMC8949640
- DOI: 10.1016/j.eclinm.2022.101362
Tocilizumab plus dexamethasone versus dexamethasone in patients with moderate-to-severe COVID-19 pneumonia: A randomised clinical trial from the CORIMUNO-19 study group
Abstract
Background: In moderate-to-severe COVID-19 pneumonia, dexamethasone (DEX) and tocilizumab (TCZ) reduce the occurrence of death and ventilatory support. We investigated the efficacy and safety of DEX+TCZ in an open randomized clinical trial.
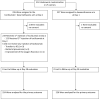
Methods: From July 24, 2020, through May 18, 2021, patients with moderate-to-severe COVID-19 pneumonia requiring oxygen (>3 L/min) were randomly assigned to receive DEX (10 mg/d 5 days tapering up to 10 days) alone or combined with TCZ (8 mg/kg IV) at day 1, possibly repeated with a fixed dose of 400 mg i.v. at day 3. The primary outcome was time from randomization to mechanical ventilation support or death up to day 14, analysed on an intent-to-treat basis using a Bayesian approach. ClinicalTrials.gov number, NCT04476979.
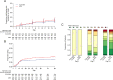
Findings: A total of 453 patients were randomized, 3 withdrew consent, 450 were analysed, of whom 226 and 224 patients were assigned to receive DEX or TCZ+DEX, respectively. At day 14, mechanical ventilation or death occurred in 32/226 (14%) and 27/224 (12%) in the DEX and TCZ+DEX arms, respectively (hazard ratio [HR] 0·85, 90% credible interval [CrI] 0·55 to 1·31). At day 14, the World health Organization (WHO) clinical progression scale (CPS) was significantly improved in the TCZ+DEX arm (OR 0·69, 95% CrI, 0·49 to 0.97). At day 28, the cumulative incidence of oxygen supply independency was 82% in the TCZ+DEX arms and 72% in the DEX arm (HR 1·36, 95% CI 1·11 to 1·67). On day 90, 24 deaths (11%) were observed in the DEX arm and 18 (8%) in the TCZ+DEX arm (HR 0·77, 95% CI 0·42-1·41). Serious adverse events were observed in 25% and 21% in DEX and TCZ+DEX arms, respectively.
Interpretation: Mechanical ventilation need and mortality were not improved with TCZ+DEX compared with DEX alone. The safety of both treatments was similar. However, given the wide confidence intervals for the estimate of effect, definitive interpretation cannot be drawn.
Funding: Programme Hospitalier de Recherche Clinique [PHRC COVID-19-20-0151, PHRC COVID-19-20-0029], Fondation de l'Assistance Publique - Hôpitaux de Paris (Alliance Tous Unis Contre le Virus) and from Fédération pour la Recherche Médicale" (FRM). Tocilizumab was provided by Roche.
Keywords: COVID-19; Randomized clinical trial; Tocilizumab+Dexamethasone.
© 2022 The Author(s).
Conflict of interest statement
The authors have the following interests to declare: Laurent Savale reports personal fees from Janssen and Janssen, andMSD, grants and personal fees from GSK, non-financial support from Acceleron, outside the submitted work. Gilles Pialoux has received consulting fees from Gilead, Abbvie, ViiVHealthcare, MSD and research grants from Gilead. Karine Lacombe Advisory boards in the Covid19 field of MSD, Gilead, GSK, Educational activities in the Covid19 field Chiesi, Sobi, MSD, Gilead, MSD. Xavier Lescure is COVID-19 advisor and has received grant from French ministry of health Jade Ghosn reports grants and personal fees from Gilead, personal fees from MSD, personal fees from ViiV Healthcare, personal fees from Janssen, personal fees from Astra Zeneca, outside the submitted work. Jean Marie Michot reports investigator fees from Amgen, BMS-Celgene, Roche, Sanofi, Xencor, Astex, outside the submitted work. François Raffi personal fees from Gilead Sciences, Janssen, MSD, Pfizer, Roche, ViiV Healthcare, outside the submitted work. Muriel Fartoukh Advisory board (community acquired pneumonia) of Pfizer, educational activities (community acquired pneumonia) of BioMérieux, (ARDS) of Fisher&Paykel. Marc Humbert personal fees from Acceleron, AstraZeneca, Bayer, Merck, Novartis, Roche, Sanofi, GSK, outside the submitted work. Frédéric Pene reports grants from ALEXION, personal fees from GILEAD, outside the submitted work. Gabriel Steg reports grants from Amarin, Bayer, Sanofi, Servier, consulting fees from Amarin, Amgen, AstraZeneca, Bayer, BMS, Idorsia, Merck, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Servier, honoraria for lectures from Mylan, support for travels from Astra Zeneca, Co inventor on Patent on alirocumab to reduce cardiovascular risk with all royalties to Sanofi, Participation on a Data Safety Monitoring Board or Advisory Board of Sanofi, New Amsterdam Pharma, Co founder of Bioquantis, all outside the submitted work. Philippe Ravaud has received grant from French ministry of health. All other authors have nothing to declare.
Figures
References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323:1239–1242. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical