Hydrogel-Mediated Release of TRPV1 Modulators to Fine Tune Osteoclastogenesis
- PMID: 35350319
- PMCID: PMC8945112
- DOI: 10.1021/acsomega.1c06915
Hydrogel-Mediated Release of TRPV1 Modulators to Fine Tune Osteoclastogenesis
Abstract
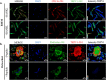
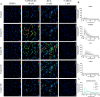
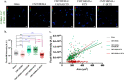
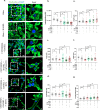
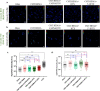
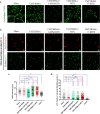
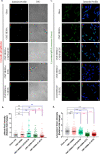
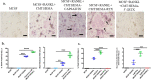
Bone defects, including bone loss due to increased osteoclast activity, have become a global health-related issue. Osteoclasts attach to the bone matrix and resorb the same, playing a vital role in bone remodeling. Ca2+ homeostasis plays a pivotal role in the differentiation and maturation of osteoclasts. In this work, we examined the role of TRPV1, a nonselective cation channel, in osteoclast function and differentiation. We demonstrate that endogenous TRPV1 is functional and causes Ca2+ influx upon activation with pharmacological activators [resiniferatoxin (RTX) and capsaicin] at nanomolar concentration, which enhances the generation of osteoclasts, whereas the TRPV1 inhibitor (5'-IRTX) reduces osteoclast differentiation. Activation of TRPV1 upregulates tartrate-resistant acid phosphatase activity and the expression of cathepsin K and calcitonin receptor genes, whereas TRPV1 inhibition reverses this effect. The slow release of capsaicin or RTX at a nanomolar concentration from a polysaccharide-based hydrogel enhances bone marrow macrophage (BMM) differentiation into osteoclasts whereas release of 5'-IRTX, an inhibitor of TRPV1, prevents macrophage fusion and osteoclast formation. We also characterize several subcellular parameters, including reactive oxygen (ROS) and nitrogen (RNS) species in the cytosol, mitochondrial, and lysosomal profiles in BMMs. ROS were found to be unaltered upon TRPV1 modulation. NO, however, had elevated levels upon RTX-mediated TRPV1 activation. Capsaicin altered mitochondrial membrane potential (ΔΨm) of BMMs but not 5'-IRTX. Channel modulation had no significant impact on cytosolic pH but significantly altered the pH of lysosomes, making these organelles less acidic. Since BMMs are precursors for osteoclasts, our findings of the cellular physiology of these cells may have broad implications in understanding the role of thermosensitive ion channels in bone formation and functions, and the TRPV1 modulator-releasing hydrogel may have application in bone tissue engineering and other biomedical sectors.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
The endovanilloid/endocannabinoid system in human osteoclasts: possible involvement in bone formation and resorption.Bone. 2009 Mar;44(3):476-84. doi: 10.1016/j.bone.2008.10.056. Epub 2008 Nov 14. Bone. 2009. PMID: 19059369
-
Sirtuin 1 inhibits TNF-α-mediated osteoclastogenesis of bone marrow-derived macrophages through both ROS generation and TRPV1 activation.Mol Cell Biochem. 2019 May;455(1-2):135-145. doi: 10.1007/s11010-018-3477-7. Epub 2018 Nov 20. Mol Cell Biochem. 2019. PMID: 30456702
-
The TRPV1 ion channel antagonist capsazepine inhibits osteoclast and osteoblast differentiation in vitro and ovariectomy induced bone loss in vivo.Bone. 2010 Apr;46(4):1089-99. doi: 10.1016/j.bone.2010.01.368. Epub 2010 Jan 22. Bone. 2010. PMID: 20096813
-
The genetic ablation or pharmacological inhibition of TRPV1 signalling is beneficial for the restoration of quiescent osteoclast activity in ovariectomized mice.Br J Pharmacol. 2014 May;171(10):2621-30. doi: 10.1111/bph.12542. Br J Pharmacol. 2014. PMID: 24308803 Free PMC article.
-
Regulators of osteoclast differentiation and cell-cell fusion.Keio J Med. 2011;60(4):101-5. doi: 10.2302/kjm.60.101. Keio J Med. 2011. PMID: 22200633 Review.
Cited by
-
Glycobiology in osteoclast differentiation and function.Bone Res. 2023 Oct 26;11(1):55. doi: 10.1038/s41413-023-00293-6. Bone Res. 2023. PMID: 37884496 Free PMC article. Review.
-
TRPV4 regulates osteoblast differentiation and mitochondrial function that are relevant for channelopathy.Front Cell Dev Biol. 2023 Jun 12;11:1066788. doi: 10.3389/fcell.2023.1066788. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37377733 Free PMC article.
-
TRP channels and cancer modulation: a voyage beyond metabolic reprogramming, oxidative stress and the advent of nanotechnologies in targeted therapy.J Exp Clin Cancer Res. 2025 Aug 14;44(1):240. doi: 10.1186/s13046-025-03495-4. J Exp Clin Cancer Res. 2025. PMID: 40813985 Free PMC article. Review.
-
The role of calcium ions and the transient receptor potential vanilloid (TRPV) channel in bone remodelling and orthodontic tooth movement.Mol Biol Rep. 2025 Mar 10;52(1):297. doi: 10.1007/s11033-025-10399-1. Mol Biol Rep. 2025. PMID: 40063148 Review.
References
-
- Feldman G. L.; Weaver D. D.; Lovrien E. W. The Fetal Trimethadione Syndrome. Am. J. Dis. Child. 1977, 131, 1389–1392. 10.1001/archpedi.1977.02120250071012. - DOI - PubMed
- Al-Sayed M. D.; Al-Zaidan H.; Albakheet A.; Hakami H.; Kenana R.; Al-Yafee Y.; Al-Dosary M.; Qari A.; Al-Sheddi T.; Al-Muheiza M.; Al-Qubbaj W.; Lakmache Y.; Al-Hindi H.; Ghaziuddin M.; Colak D.; Kaya N. Mutations in NALCN cause an autosomal-recessive syndrome with severe hypotonia, speech impairment, and cognitive delay. Am. J. Hum. Genet. 2013, 93, 721–726. 10.1016/j.ajhg.2013.08.001. - DOI - PMC - PubMed
- Plaster N. M.; Tawil R.; Tristani-Firouzi M.; Canún S.; Bendahhou S.; Tsunoda A.; Donaldson M. R.; Iannaccone S. T.; Brunt E.; Barohn R.; Clark J.; Deymeer F.; George A. L.; Fish F. A.; Hahn A.; Nitu A.; Ozdemir C.; Serdaroglu P.; Subramony S. H.; Wolfe G.; Fu Y.-H.; Ptáček L. J. Mutations in Kir2.1 Cause the Developmental and Episodic Electrical Phenotypes of Andersen’s Syndrome. Cell 2001, 105, 511–519. 10.1016/s0092-8674(01)00342-7. - DOI - PubMed
- Barel O.; Shorer Z.; Flusser H.; Ofir R.; Narkis G.; Finer G.; Shalev H.; Nasasra A.; Saada A.; Birk O. S. Mitochondrial complex III deficiency associated with a homozygous mutation in UQCRQ. Am. J. Hum. Genet. 2008, 82, 1211–1216. 10.1016/j.ajhg.2008.03.020. - DOI - PMC - PubMed
- Splawski I.; Timothy K. W.; Sharpe L. M.; Decher N.; Kumar P.; Bloise R.; Napolitano C.; Schwartz P. J.; Joseph R. M.; Condouris K.; Tager-Flusberg H.; Priori S. G.; Sanguinetti M. C.; Keating M. T. CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell 2004, 119, 19–31. 10.1016/j.cell.2004.09.011. - DOI - PubMed
-
- van der Eerden B. C. J.; Hoenderop J. G. J.; de Vries T. J.; Schoenmaker T.; Buurman C. J.; Uitterlinden A. G.; Pols H. A. P.; Bindels R. J. M.; van Leeuwen J. P. T. M. The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 17507–17512. 10.1073/pnas.0505789102. - DOI - PMC - PubMed
- Chamoux E.; Bisson M.; Payet M. D.; Roux S. TRPV-5 Mediates a Receptor Activator of NF-κB (RANK) Ligand-induced Increase in Cytosolic Ca2+ in Human Osteoclasts and Down-regulates Bone Resorption. J. Biol. Chem. 2010, 285, 25354–25362. 10.1074/jbc.m109.075234. - DOI - PMC - PubMed
- Van der Eerden B. C. J.; Weissgerber P.; Fratzl-Zelman N.; Olausson J.; Hoenderop J. G. J.; Schreuders-Koedam M.; Eijken M.; Roschger P.; De Vries T. J.; Chiba H.; Klaushofer K.; Flockerzi V.; Bindels R. J. M.; Freichel M.; van Leeuwen J. P. T. M. The transient receptor potential channel TRPV6 is dynamically expressed in bone cells but is not crucial for bone mineralization in mice. J. Cell. Physiol. 2012, 227, 1951–1959. 10.1002/jcp.22923. - DOI - PubMed
- Chen F.; Ni B.; Yang Y. O.; Ye T.; Chen A. Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity. Cell. Physiol. Biochem. 2014, 33, 796–809. 10.1159/000358653. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

