Hispolon-Loaded Liquid Crystalline Nanoparticles: Development, Stability, In Vitro Delivery Profile, and Assessment of Hepatoprotective Activity in Hepatocellular Carcinoma
- PMID: 35350323
- PMCID: PMC8945187
- DOI: 10.1021/acsomega.1c06796
Hispolon-Loaded Liquid Crystalline Nanoparticles: Development, Stability, In Vitro Delivery Profile, and Assessment of Hepatoprotective Activity in Hepatocellular Carcinoma
Abstract
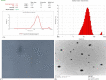
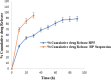
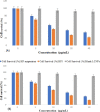
The present work describes the development and characterization of liquid crystalline nanoparticles of hispolon (HP-LCNPs) for treating hepatocellular carcinoma. HP-LCNPs were prepared by a top-down method utilizing GMO as the lipid and Pluronic F-127 as the polymeric stabilizer. The prepared formulations (HP1-HP8) were tested for long-term stability, where HP5 showed good stability with a particle size of 172.5 ± 0.3 nm, a polydispersity index (PDI) of 0.38 ± 0.31 nm, a zeta potential of -10.12 mV ± 0.05, an entrapment efficiency of 86.81 ± 2.5%, and a drug loading capacity of 12.51 ± 1.12%. Optical photomicrography and transmission electron microscopy images demonstrated a consistent, low degree of aggregation and a spherical shape of LCNPs. The effect of temperature and pH on the optimized formulation (HP5) indicated good stability at 45 °C and at pH between 2 and 5. In vitro gastrointestinal stability indicated no significant change in the particle size, PDI, and entrapment efficiency of the drug. The drug release study exhibited a biphasic pattern in simulated gastric fluid (pH 1.2) for 2 h and simulated intestinal fluid (pH 7.4) for up to 24 h, while the best fitting of the profile was observed with the Higuchi model, indicating the Fickian diffusion mechanism. The in vivo pharmacokinetic study demonstrated nearly 4.8-fold higher bioavailability from HP5 (AUC: 1774.3 ± 0.41 μg* h/mL) than from the HP suspension (AUC: 369.11 ± 0.11 μg* h/mL). The anticancer activity evaluation revealed a significant improvement in antioxidant parameters and serum hepatic biomarkers (SGOT, SGPT, ALP, total bilirubin, and GGT) in the diethyl nitrosamine-treated group of rats with the optimized LCNP formulation (HP5) vis-à-vis HP suspension.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Rahman M.; Almalki W. H.; Alrobaian M.; Iqbal J.; Alghamdi S.; Alharbi K. S.; Alruwaili N. K.; Hafeez A.; Shaharyar A.; Singh T.; et al. Nanocarriers-loaded with natural actives as newer therapeutic interventions for the treatmentof hepatocellular carcinoma. Expert Opin. Drug Delivery 2021, 18, 489–513. 10.1080/17425247.2021.1854223. - DOI - PubMed
-
- Rahman M.; Almalki W. H.; Afzal O.; Alfawaz Altamimi A. S.; Kazmi I.; Al-Abbasi F. A.; Choudhry H.; Alenezi S.; Barkat M. A.; Beg S.; et al. Cationic Solid Lipid Nanoparticles of Resveratrol for Hepatocellular Carcinoma Treatment: Systematic Optimization, in vitro Characterization and Preclinical Investigation. Int. J. Nanomed. 2020, 15, 9283–9299. 10.2147/ijn.s277545. - DOI - PMC - PubMed
-
- Venkateswarlu S.; Ramachandra M. S.; Sethuramu K.; Subbaraju G. V. Synthesis and antioxidant activity of hispolon, a yellow pigment from Inonotushispidius. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2002, 41, 875–877.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

