Vaginal Microbiota and Mucosal Pharmacokinetics of Tenofovir in Healthy Women Using a 90-Day Tenofovir/Levonorgestrel Vaginal Ring
- PMID: 35350436
- PMCID: PMC8957918
- DOI: 10.3389/fcimb.2022.799501
Vaginal Microbiota and Mucosal Pharmacokinetics of Tenofovir in Healthy Women Using a 90-Day Tenofovir/Levonorgestrel Vaginal Ring
Abstract
Background: A relationship between the vaginal microbiota and tenofovir (TFV) concentrations and activity after topical administration has been previously reported.
Objective: CONRAD A15-138 was a randomized, placebo-controlled Phase I study aimed at characterizing the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of TFV and levonorgestrel (LNG) administered through a vaginal ring (IVR) for 90 days. Herein, we describe changes from baseline in the vaginal microbiota with IVR use and the impact of the vaginal microbiota on mucosal TFV PK.
Methods: The study screened 68 participants and randomized 47 (37 TFV/LNG, 10 placebo), assessing the vaginal microbiota by sequencing the V3-V4 regions of 16S rRNA genes prior to IVR insertion and monthly for 3 months. Concentrations of TFV in vaginal fluid (VF), and TFV and TFV-diphosphate (TFV-DP) in vaginal tissue, and modeled PD against HIV-1 in vitro were measured before and after treatment.
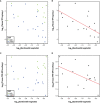
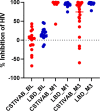
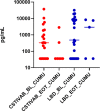
Results: There were no clinically significant changes in relative abundance of vaginal bacterial phylotypes from pre-insertion baseline at any month among active and placebo IVR users. There were no significant changes in community state type (CST) with IVR use. Participants with diverse, anaerobic CST IVA/B microbiota had higher in vivo release of TFV from the IVR compared to women with Lactobacillus-dominated (LbD) microbiota, who had expected in vivo TFV release rates. Median VF TFV concentrations were significantly higher among women with CST IVA/B microbiota in months 1 (3,135 ng/mg VF) and 2 (3,800 ng/mg). Women with LbD microbiota had significantly higher median VF TFV concentration (1,423 ng/mg) and median TFV (103 ng/mg) and TFV-DP (5,877 fmol/mg) tissue concentrations versus women with CST IVA/B microbiota at month 3. All women demonstrated a significant increase from pre-insertion baseline of in vitro HIV-1 inhibition by VF (p values <0.05). PD differences in tissue according to CST, however, were not statistically significant.
Conclusion: TFV/LNG IVR use did not change the vaginal microbiota nor increase the incidence of CST IVA/B. Vaginal microbiota, and in particular CST IVA/B, possibly through increased vaginal pH, impacted in vivo TFV release and cervicovaginal (CV) PK, but both PK and PD data suggest CV protection against HIV-1.
Clinical trial registration: ClinicalTrials.gov (#NCT03279120).
Keywords: PrEP; multipurpose prevention technology; pre-exposure (PrEP) prophylaxis; tenofovir diphosphate; vaginal microbiome.
Copyright © 2022 Thurman, Ravel, Gajer, Marzinke, Ouattara, Jacot, Peet, Clark and Doncel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

