Abrupt versus gradual smoking cessation with pre-cessation varenicline therapy for Chinese treatment-seeking smokers: A retrospective, observational, cohort study
- PMID: 35350549
- PMCID: PMC8922294
- DOI: 10.18332/tid/145993
Abrupt versus gradual smoking cessation with pre-cessation varenicline therapy for Chinese treatment-seeking smokers: A retrospective, observational, cohort study
Abstract
Introduction: This study aimed to explore the efficacy of abrupt and gradual smoking cessation with pre-cessation varenicline therapy.
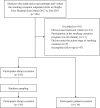
Methods: A total of 278 smokers who experienced moderate-to-severe nicotine dependence and visited a Chinese smoking cessation outpatient clinic from March 2017 to February 2021 were enrolled. This was a retrospective, observational, cohort study. Participants were divided into two groups by the cessation strategy they received: the abrupt cessation group (n=139, tobacco was not controlled during the first 3 weeks before the target cessation date and smoking was entirely discontinued on the 22nd day) and the gradual cessation group (n=139, tobacco was gradually reduced in the first 3 weeks before the target cessation date and smoking was discontinued on the 22nd day). The abstinence rates were compared between groups (7-day point prevalence abstinence rates at 1, 3 and 6 months post-treatment; and 1-month and 3-month continuous abstinence rates of 6-month follow-up). Possible factors that influence efficacy, reasons for smoking cessation failure, and associated adverse events were also analyzed.
Results: No significant difference in the 7-day point prevalence abstinence rates at 1, 3 and 6 months post-treatment was observed between the groups (p>0.05). The 1-month continuous abstinence rate of the gradual cessation group was higher than that of the abrupt cessation group (51.1% vs 31.7%; χ2=10.812, p=0.001). The 3-month continuous abstinence rate of the gradual cessation group was also higher than that of the abrupt cessation group (42.4% vs 27.3%; χ2=6.983, p=0.008). Abrupt cessation was a risk factor for successful smoking cessation than gradual cessation (AOR=2.39; 95% CI: 1.15-3.85, p=0.013),the motivation of 'prevention and treatment of own diseases' reduced the risk of incomplete abstinence (AOR=0.87; 95% CI: 0.38-0.99, p=0.049). The incidence of adverse events was higher in the abrupt cessation group than in the gradual cessation group. The incidence rates of nausea and insomnia were statistically significant differences.
Conclusions: Compared with abrupt cessation, gradual smoking cessation with pre-cessation varenicline therapy produced higher abstinence rates and relatively milder withdrawal symptoms.
Keywords: abrupt cessation; adverse events; efficacy of smoking cessation therapies; gradual cessation; varenicline.
© 2022 Zhu N. et al.
Conflict of interest statement
The authors have each completed and submitted an ICMJE form for disclosure of potential conflicts of interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. All the authors report that since the initial planning of the work and in the past 36 months, the study was supported by the Science and Technology Innovation 2025 Major Project of Ningbo (2019B10037).
Similar articles
-
[Smoking abstinence rate and its associated factors between abrupt and gradual smoking cessation].Zhonghua Jie He He Hu Xi Za Zhi. 2017 Dec 12;40(12):898-902. doi: 10.3760/cma.j.issn.1001-0939.2017.12.006. Zhonghua Jie He He Hu Xi Za Zhi. 2017. PMID: 29224298 Clinical Trial. Chinese.
-
Varenicline for Gradual Versus Abrupt Smoking Cessation in Poorly Motivated Smokers With COPD: A Prematurely Terminated Randomized Controlled Trial.Chronic Obstr Pulm Dis. 2022 Oct 26;9(4):486-499. doi: 10.15326/jcopdf.2022.0305. Chronic Obstr Pulm Dis. 2022. PMID: 35877930 Free PMC article.
-
Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial.BMC Med. 2016 Jun 7;14:80. doi: 10.1186/s12916-016-0626-2. BMC Med. 2016. PMID: 27233840 Free PMC article. Clinical Trial.
-
Varenicline: a first-line treatment option for smoking cessation.Clin Ther. 2009 Mar;31(3):463-91. doi: 10.1016/j.clinthera.2009.03.021. Clin Ther. 2009. PMID: 19393839 Review.
-
Varenicline: a novel pharmacotherapy for smoking cessation.Drugs. 2009 Jul 9;69(10):1319-38. doi: 10.2165/00003495-200969100-00003. Drugs. 2009. PMID: 19583451 Review.
References
LinkOut - more resources
Full Text Sources