In vitro Evaluation of Antiviral Efficacy of a Standardized Hydroalcoholic Extract of Poplar Type Propolis Against SARS-CoV-2
- PMID: 35350622
- PMCID: PMC8958028
- DOI: 10.3389/fmicb.2022.799546
In vitro Evaluation of Antiviral Efficacy of a Standardized Hydroalcoholic Extract of Poplar Type Propolis Against SARS-CoV-2
Abstract
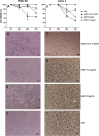
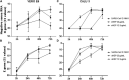
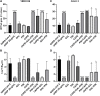
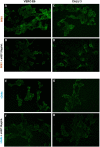
Except for specific vaccines and monoclonal antibodies, effective prophylactic or post-exposure therapeutic treatments are currently limited for COVID-19. Propolis, a honeybee's product, has been suggested as a potential candidate for treatment of COVID-19 for its immunomodulatory properties and for its powerful activity against various types of viruses, including common coronaviruses. However, direct evidence regarding the antiviral activities of this product still remains poorly documented. VERO E6 and CALU3 cell lines were infected with SARS-CoV-2 and cultured in the presence of 12.5 or 25 μg/ml of a standardized Hydroalcoholic Extract acronym (sHEP) of Eurasian poplar type propolis and analyzed for viral RNA transcription, for cell damage by optical and electron microscopy, and for virus infectivity by viral titration at 2, 24, 48, and 72 h post-infection. The three main components of sHEP, caffeic acid phenethyl ester, galangin, and pinocembrin, were tested for the antiviral power, either alone or in combination. On both cell lines, sHEP showed significant effects mainly on CALU3 up to 48 h, i.e., some protection from cytopathic effects and consistent reduction of infected cell number, fewer viral particles inside cellular vesicles, reduction of viral titration in supernatants, dramatic drop of N gene negative sense RNA synthesis, and lower concentration of E gene RNA in cell extracts. Interestingly, pre-treatment of cells with sHEP before virus inoculation induced these same effects described previously and was not able to block virus entry. When used in combination, the three main constituents of sHEP showed antiviral activity at the same levels of sHEP. sHEP has a remarkable ability to hinder the replication of SARS-CoV-2, to limit new cycles of infection, and to protect host cells against the cytopathic effect, albeit with rather variable results. However, sHEP do not block the virus entry into the cells. The antiviral activity observed with the three main components of sHEP used in combination highlights that the mechanism underlying the antiviral activity of sHEP is probably the result of a synergistic effect. These data add further emphasis on the possible therapeutic role of this special honeybee's product as an adjuvant to official treatments of COVID-19 patients for its direct antiviral activity.
Keywords: COVID-19; COVID-19 treatment; SARS-CoV-2; pandemic (COVID-19); propolis.
Copyright © 2022 Sberna, Biagi, Marafini, Nardacci, Biava, Colavita, Piselli, Miraldi, D’Offizi, Capobianchi and Amendola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Berretta A. A., Arruda C., Miguel F., Baptista N., Nascimento A., Marquele- Oliveira F., et al. (2017). “Functional properties of Brazilian propolis: from chemical composition until the market,” in Superfood and Functional Food - An Overview of Their Processing and Utilization, ed. Waisundara V. (London: Intech Open; ), 55–98.
LinkOut - more resources
Full Text Sources
Miscellaneous

