QT prolongation in the STREAM Stage 1 Trial
- PMID: 35351238
- PMCID: PMC8982645
- DOI: 10.5588/ijtld.21.0403
QT prolongation in the STREAM Stage 1 Trial
Abstract
BACKGROUND: STREAM (Standardized Treatment Regimen of Anti-TB Drugs for Patients with MDR-TB) Stage 1 demonstrated non-inferior efficacy of a shortened regimen (the Short regimen) for rifampicin-resistant TB (RR-TB) compared to the contemporaneous WHO-recommended regimen. This regimen included moxifloxacin and clofazimine, known to cause QT prolongation, and severe prolongation was more common on the Short regimen. Here we investigate risk factors for QT prolongation with the Short regimen.METHODS: Data from patients prescribed the Short regimen (n = 282) were analysed to identify risk factors for severe QT prolongation (QT/QTcF ≥500 ms or ≥60 ms increase in QTcF from baseline).RESULTS: Of the 282 patients on the Short regimen, 94 (33.3%) developed severe QT prolongation: 31 QT/QTcF ≥500 ms; 92 experienced ≥60 ms QTcF increase from baseline. The median time to QT/QTcF ≥500 ms was 20 weeks (IQR 8-28), and the time to ≥60 ms increase from baseline was 18 weeks (IQR 8-28). Prolongation ≥500 ms was most frequent in patients from Mongolia (10/22, 45.5%) compared with 3.5-11.9% at other sites, P < 0.001. Higher baseline QTcF increased risk of prolongation to ≥500 ms (QTcF ≥400 ms: OR 5.99, 95% CI 2.04-17.62).CONCLUSION: One third of patients on the Short regimen developed severe QT prolongation. QT/QTcF ≥500 ms was more common in patients from Mongolia and in those with a higher baseline QTcF, which may have implications for implementation of treatment.
CONTEXTE :: L’essai clinique STREAM (Standardized Treatment Regimen of Anti-TB Drugs for Patients with MDR-TB)Étape 1 a démontré l’efficacité non inférieure d’un schéma plus court pour le traitement de la TB résistante à la rifampicine (RR-TB) par rapport au schéma recommandé par l’OMS. Ce schéma comprenait moxifloxacine et clofazimine, connues pour provoquer un allongement de l’intervalle QT. Les cas graves d’allongement de l’intervalle QT étaient plus fréquents sous schéma court. Nous avons analysé les facteurs de risque d’allongement de l’intervalle QT chez les patients sous schéma court.
MÉTHODES :: Les données des patients randomisés sous schéma court (n = 282) ont été analysées pour identifier les facteurs de risque de grave allongement de l’intervalle QT (QT/QTcF ≥500 ms ou augmentation ≥60 ms de l’intervalle QTcF par rapport à l’inclusion).
RÉSULTATS :: Ont été associés 94 (33,3%) patients sous schéma court à un grave allongement de l’intervalle QT : 31 cas de QT/QTcF ≥500 ms ; 92 cas d’augmentation ≥60 ms de l’intervalle QTcF par rapport à l’inclusion. Le temps médian avant QT/QTcF ≥500 ms était de 20 (IQR 8–28) semaines et de 18 semaines (IQR 8–28) avant augmentation ≥60 ms par rapport à l’inclusion. Un allongement ≥500 ms était plus fréquent chez les patients de Mongolie (10/22, 45,5%), par rapport à 3,5–11,9% pour les autres pays (P < 0,001). Un intervalle QTcF plus élevé à l’inclusion augmentait le risque d’allongement ≥500 ms (QTcF ≥400 ms : OR 5,99, IC 95% 2,04–17,62).
CONCLUSION :: Un tiers des patients sous schéma court ont été associés à un grave allongement de l’intervalle QT. Un intervalle QT/QTcF ≥500 ms était plus fréquent chez les patients de Mongolie et chez ceux dont l’intervalle QTcF était plus élevé à l’inclusion, ce qui pourrait impacter l’instauration du traitement.
Conflict of interest statement
Conflicts of interest: none declared.
Figures


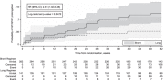
Comment in
-
QT prolongation for old and new drugs: how much should we really worry?Int J Tuberc Lung Dis. 2022 Apr 1;26(4):298-301. doi: 10.5588/ijtld.22.0072. Int J Tuberc Lung Dis. 2022. PMID: 35351233 No abstract available.
References
-
- Monedero-Recuero I, et al. QTc and anti-tuberculosis drugs: A perfect storm or a tempest in a teacup? Review of evidence and a risk assessment. Int J Tuberc Lung Dis. 2018;22(12):1411–1421. - PubMed
-
- Nunn AJ, et al. A trial of a shorter regimen for rifampinresistant tuberculosis. N Engl J Med. 2019;380(13):1201–1213. - PubMed
-
- World Health Organization Geneva, Switzerland: WHO; 2011. Guidelines for the programmatic management of drug-resistance tuberculosis; pp. 1–44.
-
- Van Deun A et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–692. - PubMed
-
- World Health Organization Geneva, Switzerland: WHO; 2011. Annex 2. WHO good manufacturing practices for pharmaceutical products: main principles; pp. 77–135.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

