Macrophage inhibitory cytokine-1 in cancer: Beyond the cellular phenotype
- PMID: 35351601
- PMCID: PMC9088220
- DOI: 10.1016/j.canlet.2022.215664
Macrophage inhibitory cytokine-1 in cancer: Beyond the cellular phenotype
Abstract
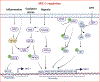
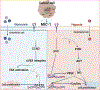
Despite technological advances in diagnostic abilities and improved treatment methods, the burden of cancers remains high, leading to significant morbidity and mortality. One primary reason is that cancer cell secretory factors modulate the tumor microenvironment, supporting tumor growth and circumvents anticancer activities of conventional therapies. Macrophage inhibitory cytokine-1 (MIC-1) is a pleiotropic cytokine elevated in various cancers. MIC-1 regulates various cancer hallmarks, including sustained proliferation, tumor-promoting inflammation, avoiding immune destruction, inducing invasion, metastasis, angiogenesis, and resisting cell death. Despite these facts, the molecular regulation and downstream signaling of MIC-1 in cancer remain elusive, partly because its receptor (GFRAL) was unknown until recently. Binding of MIC-1 to GFRAL recruits the coreceptor tyrosine kinase RET to execute its downstream signaling. So far, studies have shown that GFRAL expression is restricted to the brain stem and is responsible for MIC-1/GFRAL/RET-mediated metabolic disorders. Nevertheless, abundant levels of MIC-1 expression have been reported in all cancer types and have been proposed as a surrogate biomarker. Given the ubiquitous expression of MIC-1 in cancers, it is crucial to understand both upstream regulation and downstream MIC-1/GFRAL/RET signaling in cancer hallmark traits.
Keywords: Cancer; Cancer hallmarks; Immunosurveillance; MIC-1/GFRAL/RET signaling; Surrogate biomarker.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
SKB is a founding member of Sanguine Diagnostics and Therapeutics, Inc. Other authors have no conflicts of interest.
Figures



References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J Clin, 71 (2021) 209–249. - PubMed
-
- Tsai VWW, Husaini Y, Sainsbury A, Brown DA, Breit SN, The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases, Cell Metab, 28 (2018) 353–368. - PubMed
-
- Zhao L, Lee BY, Brown DA, Molloy MP, Marx GM, Pavlakis N, Boyer MJ, Stockler MR, Kaplan W, Breit SN, Sutherland RL, Henshall SM, Horvath LG, Identification of candidate biomarkers of therapeutic response to docetaxel by proteomic profiling, Cancer Res, 69 (2009) 7696–7703. - PubMed
-
- Zhang Y, Hua W, Niu LC, Li SM, Wang YM, Shang L, Zhang C, Li WN, Wang R, Chen BL, Xin XY, Zhang YQ, Wang J, Elevated growth differentiation factor 15 expression predicts poor prognosis in epithelial ovarian cancer patients, Tumour Biol, 37 (2016) 9423–9431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

