New Metabolic Alterations and A Predictive Marker Pipecolic Acid in Sera for Esophageal Squamous Cell Carcinoma
- PMID: 35351627
- PMCID: PMC9880896
- DOI: 10.1016/j.gpb.2021.08.016
New Metabolic Alterations and A Predictive Marker Pipecolic Acid in Sera for Esophageal Squamous Cell Carcinoma
Abstract
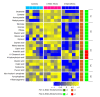
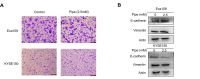
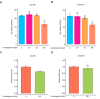
Esophageal squamous cell carcinoma (ESCC) is a major histological subtype of esophageal cancer with a poor prognosis. Although several serum metabolomic investigations have been reported, ESCC tumor-associated metabolic alterations and predictive biomarkers in sera have not been defined. Here, we enrolled 34 treatment-naive patients with ESCC and collected their pre- and post-esophagectomy sera together with the sera from 34 healthy volunteers for a metabolomic survey. Our comprehensive analysis identified ESCC tumor-associated metabolic alterations as represented by a panel of 12 serum metabolites. Notably, postoperative abrosia and parenteral nutrition substantially perturbed the serum metabolome. Furthermore, we performed an examination using sera from carcinogen-induced mice at the dysplasia and ESCC stages and identified three ESCC tumor-associated metabolites conserved between mice and humans. Notably, among these metabolites, the level of pipecolic acid was observed to be progressively increased in mouse sera from dysplasia to cancerization, and it could be used to accurately discriminate between mice at the dysplasia stage and healthy control mice. Furthermore, this metabolite is essential for ESCC cells to restrain oxidative stress-induced DNA damage and cell proliferation arrest. Together, this study revealed a panel of 12 ESCC tumor-associated serum metabolites with potential for monitoring therapeutic efficacy and disease relapse, presented evidence for refining parenteral nutrition composition, and highlighted serum pipecolic acid as an attractive biomarker for predicting ESCC tumorigenesis.
Keywords: Esophageal squamous cell carcinoma; Esophagectomy; Pipecolic acid; Predictive potential; Serum metabolome.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Figures












References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Lagergren J., Smyth E., Cunningham D., Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–2396. - PubMed
-
- Arnold M., Soerjomataram I., Ferlay J., Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. - PubMed
-
- Raghu Subramanian C., Triadafilopoulos G. Diagnosis and therapy of esophageal squamous cell dysplasia and early esophageal squamous cell cancer. Gastroenterol Rep. 2017;5:247–257.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

