Host liver-derived extracellular vesicles deliver miR-142a-3p induces neutrophil extracellular traps via targeting WASL to block the development of Schistosoma japonicum
- PMID: 35351657
- PMCID: PMC9092393
- DOI: 10.1016/j.ymthe.2022.03.016
Host liver-derived extracellular vesicles deliver miR-142a-3p induces neutrophil extracellular traps via targeting WASL to block the development of Schistosoma japonicum
Abstract
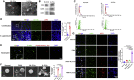
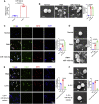
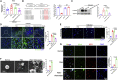
Schistosomiasis is an important neglected tropical disease. Interactions between the host immune system and schistosomes are complex. Neutrophils contribute to clearance of large pathogens primarily by releasing neutrophil extracellular traps (NETs). However, the functional role of NETs in clearing schistosomes remains unclear. Herein, we report that extracellular vesicles (EVs) derived from the liver of Schistosoma japonicum-infected mice (IL-EVs) induce NET release by delivering miR-142a-3p to target WASL and block the development of S. japonicum. WASL knockout accelerated the formation of NETs that blocked further development of S. japonicum. miR-142a-3p and NETs upregulated the expression of CCL2, which recruits macrophages that block S. japonicum development. However, S. japonicum inhibited NET formation in wild-type mice by upregulating host interleukin-10 (IL-10) expression. In contrast, in WASL knockout mice, IL-10 expression was downregulated, and S. japonicum-mediated inhibition of NET formation was significantly reduced. IL-EV-mediated induction of NET formation is thus an anti-schistosome response that can be counteracted by S. japonicum. These findings suggest that IL-EV-mediated induction of NET formation plays a key role in schistosome infection and that WASL is a potential therapeutic target in schistosomiasis and other infectious diseases.
Keywords: Schistosoma japonicum; WASL; extracellular vesicles; miR-142a-3p; neutrophil extracellular traps.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






Similar articles
-
Schistosome egg-derived extracellular vesicles deliver Sja-miR-71a inhibits host macrophage and neutrophil extracellular traps via targeting Sema4D.Cell Commun Signal. 2023 Dec 21;21(1):366. doi: 10.1186/s12964-023-01395-8. Cell Commun Signal. 2023. PMID: 38129877 Free PMC article.
-
Characterization of MicroRNA Cargo of Extracellular Vesicles Isolated From the Plasma of Schistosoma japonicum-Infected Mice.Front Cell Infect Microbiol. 2022 Feb 28;12:803242. doi: 10.3389/fcimb.2022.803242. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35295754 Free PMC article.
-
COVID-19 patient serum-derived extracellular vesicles deliver miR-20b-5p induces neutrophil extracellular traps.Cell Commun Signal. 2025 Feb 17;23(1):93. doi: 10.1186/s12964-025-02095-1. Cell Commun Signal. 2025. PMID: 39962581 Free PMC article.
-
Emerging roles for extracellular vesicles in Schistosoma infection.Acta Trop. 2022 Aug;232:106467. doi: 10.1016/j.actatropica.2022.106467. Epub 2022 Apr 12. Acta Trop. 2022. PMID: 35427535 Review.
-
Protein Kinases: Potential Drug Targets Against Schistosoma japonicum.Front Cell Infect Microbiol. 2021 Jul 1;11:691757. doi: 10.3389/fcimb.2021.691757. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34277472 Free PMC article. Review.
Cited by
-
Schistosome egg-derived extracellular vesicles deliver Sja-miR-71a inhibits host macrophage and neutrophil extracellular traps via targeting Sema4D.Cell Commun Signal. 2023 Dec 21;21(1):366. doi: 10.1186/s12964-023-01395-8. Cell Commun Signal. 2023. PMID: 38129877 Free PMC article.
-
MicroRNAs in infectious diseases: potential diagnostic biomarkers and therapeutic targets.Clin Microbiol Rev. 2023 Dec 20;36(4):e0001523. doi: 10.1128/cmr.00015-23. Epub 2023 Nov 1. Clin Microbiol Rev. 2023. PMID: 37909789 Free PMC article. Review.
-
Roquin-1 resolves sepsis-associated acute liver injury by regulating inflammatory profiles via miRNA cargo in extracellular vesicles.iScience. 2023 Jul 12;26(8):107295. doi: 10.1016/j.isci.2023.107295. eCollection 2023 Aug 18. iScience. 2023. PMID: 37554446 Free PMC article.
-
Egg-driven immunosuppression and granuloma zonation in Peyer's patches of mice with Schistosoma japonicum infection.Front Cell Infect Microbiol. 2025 Apr 29;15:1587166. doi: 10.3389/fcimb.2025.1587166. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40365538 Free PMC article.
-
Inflammatory microRNAs in cardiovascular pathology: another brick in the wall.Front Immunol. 2023 May 18;14:1196104. doi: 10.3389/fimmu.2023.1196104. eCollection 2023. Front Immunol. 2023. PMID: 37275892 Free PMC article. Review.
References
-
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

