Hydrogen phosphate selectively induces MDA MB 231 triple negative breast cancer cell death in vitro
- PMID: 35351930
- PMCID: PMC8964734
- DOI: 10.1038/s41598-022-09299-2
Hydrogen phosphate selectively induces MDA MB 231 triple negative breast cancer cell death in vitro
Abstract
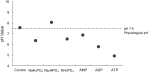
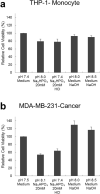
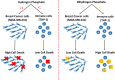
Phosphate ions are the most abundant anions inside the cells, and they are increasingly gaining attention as key modulators of cellular function and gene expression. However, little is known about the effect of inorganic phosphate ions on cancer cells, particularly breast cancer cells. Here, we investigated the toxicity of different phosphate compounds to triple-negative human breast cancer cells, particularly, MDA-MB-231, and compared it to that of human monocytes, THP-1. We found that, unlike dihydrogen phosphate (H2PO4-), hydrogen phosphate (HPO42-) at 20 mM or lower concentrations induced breast cancer cell death more than immune cell death, mainly via apoptosis. We correlate this effect to the fact that phosphate in the form of HPO42- raises pH levels to alkaline levels which are not optimum for transport of phosphate into cancer cells. The results in this study highlight the importance of further exploring hydrogen phosphate (HPO42-) as a potential therapeutic for the treatment of breast cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Lacerda-Abreu MA, et al. H+-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2019;1865:2180–2188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

