Calcium Imaging and Electrophysiology of hippocampal Activity under Anesthesia and natural Sleep in Mice
- PMID: 35351935
- PMCID: PMC8964694
- DOI: 10.1038/s41597-022-01244-2
Calcium Imaging and Electrophysiology of hippocampal Activity under Anesthesia and natural Sleep in Mice
Abstract
The acute effects of anesthesia and their underlying mechanisms are still not fully understood. Thus, comprehensive analysis and efficient generalization require their description in various brain regions. Here we describe a large-scale, annotated collection of 2-photon calcium imaging data and multi-electrode, extracellular electrophysiological recordings in CA1 of the murine hippocampus under three distinct anesthetics (Isoflurane, Ketamine/Xylazine and Medetomidine/Midazolam/Fentanyl), during natural sleep, and wakefulness. We cover several aspects of data quality standardization and provide a set of tools for autonomous validation, along with analysis workflows for reuse and data exploration. The datasets described here capture various aspects of neural activity in hundreds of pyramidal cells at single cell resolution. In addition to relevance for basic biological research, the dataset may find utility in computational neuroscience as a benchmark for models of anesthesia and sleep.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




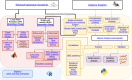
References
-
- Pachitariu, M. et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. 061507, 10.1101/061507(2017).
Publication types
MeSH terms
Substances
Grants and funding
- CSC 201606210129/National Science Foundation of China | Joint Research Fund for Overseas Chinese Scholars and Scholars in Hong Kong and Macao
- 178316478 - B5/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 178316478 - B8/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ERC-2015-CoG-681577/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- MSCA-ITN-H2020-860563/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

