Förster resonance energy transfer biosensors for fluorescence and time-gated luminescence analysis of rac1 activity
- PMID: 35351946
- PMCID: PMC8964680
- DOI: 10.1038/s41598-022-09364-w
Förster resonance energy transfer biosensors for fluorescence and time-gated luminescence analysis of rac1 activity
Abstract
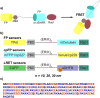
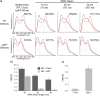
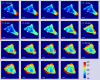
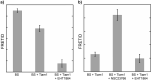
Genetically encoded, Förster resonance energy transfer (FRET) biosensors enable live-cell optical imaging of signaling molecules. Small conformational changes often limit the dynamic range of biosensors that combine fluorescent proteins (FPs) and sensing domains into a single polypeptide. To address this, we developed FRET and lanthanide-based FRET (LRET) biosensors of Rac1 activation with two key features that enhance sensitivity and dynamic range. For one, alpha helical linker domains separate FRET partners and ensure a large conformational change and FRET increase when activated Rac1 at the biosensor C-terminus interacts with an amino-terminal Rac binding domain. Incorporation of a luminescent Tb(III) complex with long (~ ms) excited state lifetime as a LRET donor enabled time-gated luminescence measurements of Rac1 activity in cell lysates. The LRET dynamic range increased with ER/K linker length up to 1100% and enabled robust detection of Rac1 inhibition in 96-well plates. The ER/K linkers had a less pronounced, but still significant, effect on conventional FRET biosensors (with FP donors and acceptors), and we were able to dynamically image Rac1 activation at cell edges using fluorescence microscopy. The results herein highlight the potential of FRET and LRET biosensors with ER/K linkers for cell-based imaging and screening of protein activities.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

