Detection of signs of disease in external photographs of the eyes via deep learning
- PMID: 35352000
- PMCID: PMC8963675
- DOI: 10.1038/s41551-022-00867-5
Detection of signs of disease in external photographs of the eyes via deep learning
Abstract
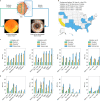
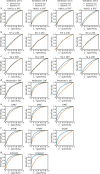
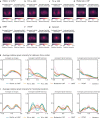
Retinal fundus photographs can be used to detect a range of retinal conditions. Here we show that deep-learning models trained instead on external photographs of the eyes can be used to detect diabetic retinopathy (DR), diabetic macular oedema and poor blood glucose control. We developed the models using eye photographs from 145,832 patients with diabetes from 301 DR screening sites and evaluated the models on four tasks and four validation datasets with a total of 48,644 patients from 198 additional screening sites. For all four tasks, the predictive performance of the deep-learning models was significantly higher than the performance of logistic regression models using self-reported demographic and medical history data, and the predictions generalized to patients with dilated pupils, to patients from a different DR screening programme and to a general eye care programme that included diabetics and non-diabetics. We also explored the use of the deep-learning models for the detection of elevated lipid levels. The utility of external eye photographs for the diagnosis and management of diseases should be further validated with images from different cameras and patient populations.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
B.B., A.M., N.K., G.S.C., L.P., D.R.W., A.V., N.H. and Y.L. are employees of Google LLC, own Alphabet stock and are co-inventors on patents (in various stages) for machine learning using medical images. I.T. is a consultant of Google LLC. J.C. is the CEO of EyePACS Inc. A.Y.M. declares no competing interests.
Figures




Comment in
-
Eyeing severe diabetes upfront.Nat Biomed Eng. 2022 Dec;6(12):1321-1322. doi: 10.1038/s41551-022-00879-1. Nat Biomed Eng. 2022. PMID: 35411115 No abstract available.
Similar articles
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2015 Jan 7;1(1):CD008081. doi: 10.1002/14651858.CD008081.pub3. Cochrane Database Syst Rev. 2015. PMID: 25564068 Free PMC article.
-
Optical coherence tomography (OCT) for detection of macular oedema in patients with diabetic retinopathy.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD008081. doi: 10.1002/14651858.CD008081.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Jan 07;1:CD008081. doi: 10.1002/14651858.CD008081.pub3. PMID: 21735421 Updated.
-
Artificial intelligence for diagnosing exudative age-related macular degeneration.Cochrane Database Syst Rev. 2024 Oct 17;10(10):CD015522. doi: 10.1002/14651858.CD015522.pub2. Cochrane Database Syst Rev. 2024. PMID: 39417312
-
Diabetic retinopathy detection from fundus images: A wide survey from grading to segmentation of lesions.Comput Biol Med. 2025 Sep;196(Pt B):110715. doi: 10.1016/j.compbiomed.2025.110715. Epub 2025 Jul 18. Comput Biol Med. 2025. PMID: 40683101 Review.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
Cited by
-
Deep learning for predicting circular retinal nerve fiber layer thickness from fundus photographs and diagnosing glaucoma.Heliyon. 2024 Jun 28;10(13):e33813. doi: 10.1016/j.heliyon.2024.e33813. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39040392 Free PMC article.
-
Predicting demographics from meibography using deep learning.Sci Rep. 2022 Sep 20;12(1):15701. doi: 10.1038/s41598-022-18933-y. Sci Rep. 2022. PMID: 36127431 Free PMC article.
-
Retinal Imaging-Based Oculomics: Artificial Intelligence as a Tool in the Diagnosis of Cardiovascular and Metabolic Diseases.Biomedicines. 2024 Sep 23;12(9):2150. doi: 10.3390/biomedicines12092150. Biomedicines. 2024. PMID: 39335664 Free PMC article. Review.
-
Design and Usability Study of a Point of Care mHealth App for Early Dry Eye Screening and Detection.J Clin Med. 2023 Oct 12;12(20):6479. doi: 10.3390/jcm12206479. J Clin Med. 2023. PMID: 37892616 Free PMC article.
-
Oculomics: A Crusade Against the Four Horsemen of Chronic Disease.Ophthalmol Ther. 2024 Jun;13(6):1427-1451. doi: 10.1007/s40123-024-00942-x. Epub 2024 Apr 17. Ophthalmol Ther. 2024. PMID: 38630354 Free PMC article. Review.
References
-
- Cheung, C. Y. et al. A deep-learning system for the assessment of cardiovascular disease risk via the measurement of retinal-vessel calibre. Nat. Biomed. Eng. 10.1038/s41551-020-00626-4 (2020). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

