Intranasal administration of a single dose of a candidate live attenuated vaccine derived from an NSP16-deficient SARS-CoV-2 strain confers sterilizing immunity in animals
- PMID: 35352010
- PMCID: PMC8961489
- DOI: 10.1038/s41423-022-00855-4
Intranasal administration of a single dose of a candidate live attenuated vaccine derived from an NSP16-deficient SARS-CoV-2 strain confers sterilizing immunity in animals
Abstract
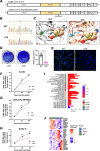
Live attenuated vaccines might elicit mucosal and sterilizing immunity against SARS-CoV-2 that the existing mRNA, adenoviral vector and inactivated vaccines fail to induce. Here, we describe a candidate live attenuated vaccine strain of SARS-CoV-2 in which the NSP16 gene, which encodes 2'-O-methyltransferase, is catalytically disrupted by a point mutation. This virus, designated d16, was severely attenuated in hamsters and transgenic mice, causing only asymptomatic and nonpathogenic infection. A single dose of d16 administered intranasally resulted in sterilizing immunity in both the upper and lower respiratory tracts of hamsters, thus preventing viral spread in a contact-based transmission model. It also robustly stimulated humoral and cell-mediated immune responses, thus conferring full protection against lethal challenge with SARS-CoV-2 in a transgenic mouse model. The neutralizing antibodies elicited by d16 effectively cross-reacted with several SARS-CoV-2 variants. Secretory immunoglobulin A was detected in the blood and nasal wash of vaccinated mice. Our work provides proof-of-principle evidence for harnessing NSP16-deficient SARS-CoV-2 for the development of live attenuated vaccines and paves the way for further preclinical studies of d16 as a prototypic vaccine strain, to which new features might be introduced to improve safety, transmissibility, immunogenicity and efficacy.
Keywords: 2′-O-methyltransferase; Live attenuated vaccine; Mucosal immunity; NSP16; Sterilizing immunity; T-cell response.
© 2022. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
A live attenuated SARS-CoV-2 vaccine constructed by dual inactivation of NSP16 and ORF3a.EBioMedicine. 2025 Apr;114:105662. doi: 10.1016/j.ebiom.2025.105662. Epub 2025 Mar 24. EBioMedicine. 2025. PMID: 40132472 Free PMC article.
-
A Bacteriophage-Based, Highly Efficacious, Needle- and Adjuvant-Free, Mucosal COVID-19 Vaccine.mBio. 2022 Aug 30;13(4):e0182222. doi: 10.1128/mbio.01822-22. Epub 2022 Jul 28. mBio. 2022. PMID: 35900097 Free PMC article.
-
Immunogenicity and safety of a live-attenuated SARS-CoV-2 vaccine candidate based on multiple attenuation mechanisms.Elife. 2025 Feb 11;13:RP97532. doi: 10.7554/eLife.97532. Elife. 2025. PMID: 39932490 Free PMC article.
-
An Intranasal OMV-Based Vaccine Induces High Mucosal and Systemic Protecting Immunity Against a SARS-CoV-2 Infection.Front Immunol. 2021 Dec 17;12:781280. doi: 10.3389/fimmu.2021.781280. eCollection 2021. Front Immunol. 2021. PMID: 34987509 Free PMC article.
-
Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence.Viruses. 2022 Jan 19;14(2):187. doi: 10.3390/v14020187. Viruses. 2022. PMID: 35215783 Free PMC article. Review.
Cited by
-
Monovalent vaccination with inactivated SARS-CoV-2 BA.5 protects hamsters against Omicron but not non-Omicron variants.NPJ Vaccines. 2023 Nov 20;8(1):177. doi: 10.1038/s41541-023-00776-x. NPJ Vaccines. 2023. PMID: 37985668 Free PMC article.
-
Nsp1 facilitates SARS-CoV-2 replication through calcineurin-NFAT signaling.mBio. 2024 Apr 10;15(4):e0039224. doi: 10.1128/mbio.00392-24. Epub 2024 Feb 27. mBio. 2024. PMID: 38411085 Free PMC article.
-
Meeting report of the 37th International Conference on Antiviral Research in Gold Coast, Australia, May 20-24, 2024, organized by the International Society for Antiviral Research.Antiviral Res. 2024 Dec;232:106037. doi: 10.1016/j.antiviral.2024.106037. Epub 2024 Nov 13. Antiviral Res. 2024. PMID: 39542140
-
M protein ectodomain-specific immunity restrains SARS-CoV-2 variants replication.Front Immunol. 2024 Oct 2;15:1450114. doi: 10.3389/fimmu.2024.1450114. eCollection 2024. Front Immunol. 2024. PMID: 39416782 Free PMC article.
-
Coronavirus 2'-O-methyltransferase: A promising therapeutic target.Virus Res. 2023 Oct 15;336:199211. doi: 10.1016/j.virusres.2023.199211. Epub 2023 Sep 4. Virus Res. 2023. PMID: 37634741 Free PMC article. Review.
References
-
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings – Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2012;70:1059–62. doi: 10.15585/mmwr.mm7031e2. - DOI - PMC - PubMed
-
- Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (δ) variant – National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1163–6. doi: 10.15585/mmwr.mm7034e3. - DOI - PMC - PubMed
-
- Tré-Hardy M, Cupaiolo R, Wilmet A, Antoine-Moussiaux T, Della Vecchia A, Horeanga A, et al. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: a third dose is expected. J Infect. 2021;S0163-4453:00433–3. doi: 10.1016/j.jinf.2021.08.031. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

