Pan-KRAS inhibitors suppress proliferation through feedback regulation in pancreatic ductal adenocarcinoma
- PMID: 35352018
- PMCID: PMC9525295
- DOI: 10.1038/s41401-022-00897-4
Pan-KRAS inhibitors suppress proliferation through feedback regulation in pancreatic ductal adenocarcinoma
Abstract
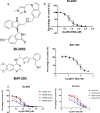
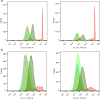
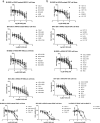
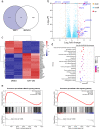
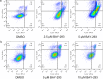
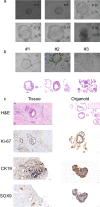
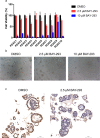
Pancreatic ductal adenocarcinoma (PDAC) is currently one of the most lethal cancers worldwide. Several basic studies have confirmed that Kirsten rat sarcoma virus (KRAS) is a key driver gene for the occurrence of PDAC, and KRAS mutations have also been found in most patients in clinical studies. In this study, two pan-KRAS inhibitors, BI-2852 and BAY-293, were chosen as chemical probes to investigate their antitumor potency in PDAC. Their inhibitory effects on KRAS activation were validated in vitro and their antiproliferative potency in PDAC cell lines were profiled, with half-maximal inhibitory concentration (IC50) values of approximately 1 μM, demonstrating the therapeutic potential of pan-KRAS inhibitors in the treatment of PDAC. However, feedback regulation in the KRAS pathway weakened inhibitor activity, which was observed by a 50 times difference in BAY-293 from in vitro activity. Furthermore, pan-KRAS inhibitors effectively inhibited cell proliferation in 3D organoids cultured from PDAC patient samples; however, there were some variations between individuals. These results provide a sufficient theoretical foundation for KRAS as a clinical therapeutic target and for the application of pan-KRAS inhibitors in the treatment of PDAC, with important scientific significance in translational medicine.
Keywords: BAY-293; KRAS; driver gene; organoid; pancreatic ductal adenocarcinoma.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The Inhibitory Response to PI3K/AKT Pathway Inhibitors MK-2206 and Buparlisib Is Related to Genetic Differences in Pancreatic Ductal Adenocarcinoma Cell Lines.Int J Mol Sci. 2022 Apr 13;23(8):4295. doi: 10.3390/ijms23084295. Int J Mol Sci. 2022. PMID: 35457111 Free PMC article.
-
Inhibitory Response to CK II Inhibitor Silmitasertib and CDKs Inhibitor Dinaciclib Is Related to Genetic Differences in Pancreatic Ductal Adenocarcinoma Cell Lines.Int J Mol Sci. 2022 Apr 16;23(8):4409. doi: 10.3390/ijms23084409. Int J Mol Sci. 2022. PMID: 35457227 Free PMC article.
-
Jorunnamycin A induces apoptosis in pancreatic ductal adenocarcinoma cells, spheroids, and patient-derived organoids by modulating KRAS-mediated survival pathways.Sci Rep. 2025 Apr 3;15(1):11376. doi: 10.1038/s41598-025-95766-5. Sci Rep. 2025. PMID: 40175514 Free PMC article.
-
Targeting KRAS for the potential treatment of pancreatic ductal adenocarcinoma: Recent advancements provide hope (Review).Oncol Rep. 2023 Nov;50(5):206. doi: 10.3892/or.2023.8643. Epub 2023 Oct 6. Oncol Rep. 2023. PMID: 37800636 Free PMC article. Review.
-
Emerging Potential Mechanism and Therapeutic Target of Ferroptosis in PDAC: A Promising Future.Int J Mol Sci. 2022 Nov 30;23(23):15031. doi: 10.3390/ijms232315031. Int J Mol Sci. 2022. PMID: 36499358 Free PMC article. Review.
Cited by
-
Recent Anti-KRASG12D Therapies: A "Possible Impossibility" for Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2025 Feb 19;17(4):704. doi: 10.3390/cancers17040704. Cancers (Basel). 2025. PMID: 40002297 Free PMC article. Review.
-
Efficacy of immune checkpoint inhibitors in advanced non-small cell lung cancer patients with rare KRAS mutations: a real-world retrospective study.Transl Lung Cancer Res. 2024 Jul 30;13(7):1672-1684. doi: 10.21037/tlcr-24-372. Epub 2024 Jul 25. Transl Lung Cancer Res. 2024. PMID: 39118889 Free PMC article.
-
Computer-Aided Drug Design Boosts RAS Inhibitor Discovery.Molecules. 2022 Sep 5;27(17):5710. doi: 10.3390/molecules27175710. Molecules. 2022. PMID: 36080477 Free PMC article. Review.
-
Targeting the 'Undruggable' Driver Protein, KRAS, in Epithelial Cancers: Current Perspective.Cells. 2023 Feb 15;12(4):631. doi: 10.3390/cells12040631. Cells. 2023. PMID: 36831298 Free PMC article. Review.
-
Defining the KRAS- and ERK-dependent transcriptome in KRAS-mutant cancers.Science. 2024 Jun 7;384(6700):eadk0775. doi: 10.1126/science.adk0775. Epub 2024 Jun 7. Science. 2024. PMID: 38843331 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

