Detection of cardiac apoptosis by [18F]ML-10 in a mouse model of permanent LAD ligation
- PMID: 35352214
- PMCID: PMC9296384
- DOI: 10.1007/s11307-022-01718-0
Detection of cardiac apoptosis by [18F]ML-10 in a mouse model of permanent LAD ligation
Abstract
Purpose: The loss of viable cardiac cells and cell death by myocardial infarction (MI) is still a significant obstacle in preventing deteriorating heart failure. Imaging of apoptosis, a defined cascade to cell death, could identify areas at risk.
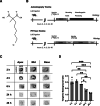
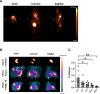
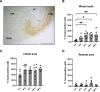
Procedures: Using 2-(5-[18F]fluoropentyl)-2-methyl-malonic acid ([18F]ML-10) in autoradiography and positron emission tomography (PET) visualized apoptosis in murine hearts after permanent ligation of the left anterior descending artery (LAD) inducing myocardial infarction (MI). 2-deoxy-2-[18F]fluoro-D-glucose ([18F]FDG) PET imaging localized the infarct area after MI. Histology by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining validated apoptosis in the heart.
Results: Accumulation of [18F]ML-10 was evident in the infarct area after permanent ligation of the LAD in autoradiography and PET imaging. Detection of apoptosis by [18F]ML-10 is in line with the defect visualized by [18F]FDG and the histological approach.
Conclusion: [18F]ML-10 could be a suitable tracer for apoptosis imaging in a mouse model of permanent LAD ligation.
Keywords: Apoptosis; Autoradiography; Cardiac positron emission tomography; Myocardial infarct; [18F]FDG; [18F]ML-10.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

