Role of nuclear factor of activated T cells in chondrogenesis osteogenesis and osteochondroma formation
- PMID: 35352320
- PMCID: PMC10024159
- DOI: 10.1007/s40618-022-01781-y
Role of nuclear factor of activated T cells in chondrogenesis osteogenesis and osteochondroma formation
Abstract
Purpose: Nuclear factor of activated T cells (NFATc) are transcription factors that play a function in the immune response and in osteoclast differentiation. In the present work, we define the function of NFATc2 in chondrogenic and osteogenic cells.
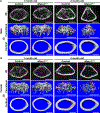
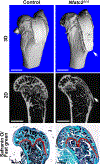
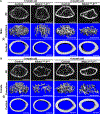
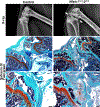
Methods: Nfatc2loxP/loxP and Nfatc1loxP/loxP;Nfatc2loxP/loxP conditional mice were crossed with Prx1-Cre transgenics to inactivate Nfatc2 singly and with Nfatc1. Femurs and vertebrae were examined by microcomputed tomography (µCT) X-Ray images and histology and analyzed for the presence of osteochondromas.
Results: µCT demonstrated that Prx1-Cre;Nfatc2∆/∆ female mice had transient osteopenia and male mice did not have a cancellous or a cortical bone phenotype when compared to control mice. In contrast, the dual inactivation of Nfatc1 and Nfatc2 in Prx1-expressing cells resulted in cancellous osteopenia and small bones at 1 month of age in both sexes. Nfatc1;Nfatc2 deleted mice exhibited a ~ 50% decrease in bone volume and connectivity. Total bone area, periosteal and endocortical bone perimeters and femoral length were reduced indicating smaller bones. As the mice matured, the shortening of the femoral length persisted, but the osteopenic phenotype resolved and cancellous femoral bone of 4-month-old Nfatc1;Nfatc2 deleted mice was not different from controls although male mice had vertebral osteopenia. In addition, Nfatc1;Nfatc2 deleted mice displayed distortion of the distal metaphysis and, as they matured, the articular presence of mineralized tumors with the appearance of osteochondromas.
Conclusion: Our studies reveal that NFATc1 and NFATc2 are necessary for optimal bone homeostasis and the suppression of osteochondroma formation.
Keywords: Chondrocytes; NFATc1; NFATc2; Osteoblasts; Osteochondroma; Skeletal homeostasis.
© 2022. The Author(s), under exclusive licence to Italian Society of Endocrinology (SIE).
Conflict of interest statement
Figures


References
-
- Crabtree GR, Olson EN(2002) NFAT signaling: choreographing the social lives of cells. Cell 109 Suppl:S67–S79. - PubMed
-
- Graef IA, Chen F, Crabtree GR (2001) NFAT signaling in vertebrate development. Curr Opin Genet Dev 11:505–512. - PubMed
-
- Ikeda F, Nishimura R, Matsubara T, Hata K, Reddy SV, Yoneda T (2006) Activation of NFAT signal in vivo leads to osteopenia associated with increased osteoclastogenesis and bone-resorbing activity. J Immunol 177:2384–2390. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

