How do I implement an outpatient program for the administration of convalescent plasma for COVID-19?
- PMID: 35352362
- PMCID: PMC9086144
- DOI: 10.1111/trf.16871
How do I implement an outpatient program for the administration of convalescent plasma for COVID-19?
Abstract
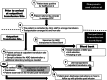
Convalescent plasma, collected from donors who have recovered from a pathogen of interest, has been used to treat infectious diseases, particularly in times of outbreak, when alternative therapies were unavailable. The COVID-19 pandemic revived interest in the use of convalescent plasma. Large observational studies and clinical trials that were executed during the pandemic provided insight into how to use convalescent plasma, whereby high levels of antibodies against the pathogen of interest and administration early within the time course of the disease are critical for optimal therapeutic effect. Several studies have shown outpatient administration of COVID-19 convalescent plasma (CCP) to be both safe and effective, preventing clinical progression in patients when administered within the first week of COVID-19. The United States Food and Drug Administration expanded its emergency use authorization (EUA) to allow for the administration of CCP in an outpatient setting in December 2021, at least for immunocompromised patients or those on immunosuppressive therapy. Outpatient transfusion of CCP and infusion of monoclonal antibody therapies for a highly transmissible infectious disease introduces nuanced challenges related to infection prevention. Drawing on our experiences with the clinical and research use of CCP, we describe the logistical considerations and workflow spanning procurement of qualified products, infrastructure, staffing, transfusion, and associated management of adverse events. The purpose of this description is to facilitate the efforts of others intent on establishing outpatient transfusion programs for CCP and other antibody-based therapies.
Keywords: COVID-19; COVID-19 serotherapy; ambulatory care; antibodies; blood transfusion; monoclonal; plasma.
© 2022 AABB.
Conflict of interest statement
Evan M. Bloch reports personal fees and non‐financial support from Terumo BCT, Grifols Diagnostic Solutions, and Abbott Laboratories, outside of the submitted work; Evan M. Bloch is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Any views or opinions that are expressed in this manuscript are those of the author's, based on his own scientific expertise and professional judgment; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of the FDA, and do not bind or otherwise obligate or commit either Advisory Committee or the Agency to the views expressed.
Figures

References
-
- Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. - PubMed
-
- Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979;2:1216–7. - PubMed
-
- Sullivan DJ, Gebo KA, Shoham S, Bloch EM, Lau B, Shenoy AG, et al. Randomized controlled trial of early outpatient COVID‐19 treatment with high‐titer convalescent plasma. medRxiv. 2021; 2021.12.10.21267485.

